
��15�֣��Ҵ������ᶼ���л�������Ҫ�Ļ���ԭ�ϡ�
��1���������У��Ҵ�������ʹ����ͭ˿���ֺ��ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ũ������£�������Ҵ�������������

ij��ѧ��ȤС���ͬѧ������װ�ý��и�������Ӧ��̽��ʵ�飺
��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ���������� ��
��С�Թ���װ����ŨNa2CO3��Һ�������ܲ�����Һ������Ϊ�˷�ֹ ��
��������ʵIJ������ʣ�
| | �Ҵ� | ���� | �������� |
| �е� | 78��0�� | 117��9�� | 77��5�� |
| ˮ���� | ���� | ���� | ���� |
| ʵ����� | �Ҵ���mL�� | ���ᣨmL�� | ����������mL�� |
| a | 2 | 2 | 1��33 |
| b | 3 | 2 | 1��57 |
| c | 4 | 2 | X |
| d | 5 | 2 | 1��76 |
| e | 2 | 3 | 1��55 |
��2CH3CH2OH + O2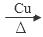 2CH3CHO + 2H2O
2CH3CHO + 2H2O
��2���ٴ��Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ�����2mL���ᡣ�ڵ�����
�۲��ܣ������������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ�Һ©�� ���Ͽڵ���
��1��57-1��76mL�� ̽��������������������������Ӱ�졣
���������������1�������У��Ҵ�������ʹ����ͭ˿���ֺ��ȣ��÷�Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH + O2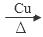 2CH3CHO + 2H2O; (2)��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ����������������Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ���ᣬ����Һ��ȴ���ټ���2mL���ᡣ��С�Թ���װ����ŨNa2CO3��Һ�������������ջӷ����Ҵ���������Ӧ�����������������ᣬ��������ζ�IJ��������������������ܽ�ȣ��Ա��ڻ����ķ����ᴿ�������ܲ�����Һ������Ϊ�˷�ֹ��������ķ������۲�����ˮ���С�Թ��е�Na2CO3��Һ������Ϊ�����������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ���С�Թ��л������ܵ�����Һ������ķ����Ƿ�Һ��ʹ�õ������Ƿ�Һ©�������ڷ���ʱ�����������������ܶȱ�ˮС�����ϲ㣬ʹ��Ӧ�ô������Ͽڵ��������ݱ������ݱ仯���ɿ�֪����������X�ķ�Χ��1��57-1��76mL��ʵ��a��ʵ��e���Ҵ���������ͬ��������������ͬ���Ҵ�������ʹ��̽����Ŀ����̽��������������������������Ӱ�졣
2CH3CHO + 2H2O; (2)��Ҫ����Թ��м�2mLŨ���ᡢ3mL�Ҵ���2mL���ᣬ����������������Թ��м���3mL�Ҵ���Ȼ������Թܱ���2mLŨ���ᣬ����Һ��ȴ���ټ���2mL���ᡣ��С�Թ���װ����ŨNa2CO3��Һ�������������ջӷ����Ҵ���������Ӧ�����������������ᣬ��������ζ�IJ��������������������ܽ�ȣ��Ա��ڻ����ķ����ᴿ�������ܲ�����Һ������Ϊ�˷�ֹ��������ķ������۲�����ˮ���С�Թ��е�Na2CO3��Һ������Ϊ�����������Ҵ��������ˮ�Ļ����Һ���ܽ�ȱȽϴܷ���С�Թ��л������ܵ�����Һ������ķ����Ƿ�Һ��ʹ�õ������Ƿ�Һ©�������ڷ���ʱ�����������������ܶȱ�ˮС�����ϲ㣬ʹ��Ӧ�ô������Ͽڵ��������ݱ������ݱ仯���ɿ�֪����������X�ķ�Χ��1��57-1��76mL��ʵ��a��ʵ��e���Ҵ���������ͬ��������������ͬ���Ҵ�������ʹ��̽����Ŀ����̽��������������������������Ӱ�졣
���㣺�����Ҵ��Ĵ�����������������ʵ������ȡ��Ӧ����Ӧԭ���������ķ����֪ʶ��


 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����е��л�������ḻ������ʳס�еȶ��Ӧ�ù㷺�������Ҵ��DZȽϳ������л��
(1)�Ҵ�����ɫ��������ζ��Һ�壬�ܶȱ�ˮ ��
(2)��ҵ������ϩ��ˮ��Ӧ���Ƶ��Ҵ����÷�Ӧ�Ļ�ѧ����ʽΪ (����д��Ӧ����)��ԭ���������� ��
(3)���������Ҵ���ͬϵ����� �������Ҵ���ͬ���칹����� ��(ѡ����)
A��
B��
C������
D���״�
E��CH3��O��CH3
F��HO��CH2CH2��OH
(4)�Ҵ��ܹ�����������Ӧ��
��46 g�Ҵ���ȫȼ������ mol������
���Ҵ���ͭ�������������¿ɱ���������Ϊ��ȩ����Ӧ�Ļ�ѧ����ʽΪ ��
������˵����ȷ���� (ѡ����ĸ)��
A���Ҵ����ܺ����Ը��������Һ����������ԭ��Ӧ
B�������ó�ɫ�������ظ������Һ���˾���Ƿ�ƺ�ݳ�
C���ƾ���ijЩ����ʹ�Ҵ�����Ϊ���ᣬ���Ǿƾͱ�����
D������ľƺ�������Ϊ�Ҵ����Ҵ����������ɵ����ᷢ��������Ӧ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ij��������Ƶĺϳɾ�����߷��Ӳ��ϵ�·�ߣ�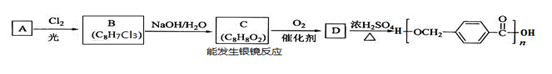
��֪��ͬһ̼ԭ�����������ǻ�ʱ�ṹ���ȶ�������ˮ����ȩ��ͪ
�����������Ϣ�ش��������⣺
��1����A�Ľṹ��ʽΪ������ ������A�Ļ�ѧ����Ϊ__________��
��2�� ��B����C�Ļ�ѧ����ʽΪ�������� ��
��3�� C��ͬ���칹���У�������FeCl3��Һ������ɫ��Ӧ�����ܷ���������Ӧ���л��ﹲ��______�֣������ں˴Ź��������г����������л���Ľṹ��ʽΪ___________��
��4�� D�Ľṹ��ʽΪ������ ��D��ijͬ���칹���к��б�����̼����������������·���ˮ�ⷴӦ�Ļ�ѧ����ʽΪ�� ���������� ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����Ƶ������÷�������ͼ��ʾ��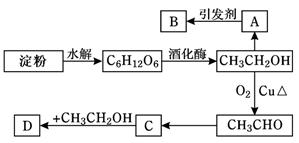
���У�A����ϩ�ܴ���ˮ����B�Ǹ߷��ӻ����D����ˮ����ζ�����ʡ���ش��������⣺
(1)��C6H12O6����������________��A�ĵ���ʽΪ__________��C�к��й��������� ��
(2) A��B��Ӧ����_________________��C��D��Ӧ����_________________
(3)д������ת���Ļ�ѧ����ʽ
��A��B�� ��
��C��D�� ��
��CH3CH2OH��CH3CHO�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��5�֣������м������ʣ��뽫����������пո��ڣ�
| A��CH2=CH-COOH�����ᣨC17H33COOH�� |
| B��12C60��ʯī |
C�� �� ��  |
| D��35Cl��37ClE���Ҵ����Ҷ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
�Ҵ�������Ϊһ���������ȼ�ϣ���Ŀǰ�����Ͽ�������Դ�ķ�չ�ص㣬���Ҿ��нϺõľ���Ч������Ч�棬�ս���Ϊ���ͺͲ��͵����Ʒ��
(1)д���Ҵ���ȫȼ�յĻ�ѧ����ʽ��______________________________��
(2)�Ҵ�ȼ��ʱ����������㣬���ܻ���CO���ɡ�����ͼװ����֤�Ҵ���ȼ�ղ�������CO��CO2��H2O��Ӧ���Ҵ���ȼ�ղ�������ͨ��(�����������ҵ�˳����װ�ñ��)________��
(3)ʵ��ʱ�ɹ۲쵽װ�â���Aƿ��ʯ��ˮ����ǡ�Aƿ��Һ��������________��Bƿ��Һ��������________��Cƿ��Һ��������_________________________��
(4)װ�â۵�������________��װ�â�����ʢ����________��������______________________��
(5)װ�â�����ʢ�Ĺ���ҩƷ��________����������֤�IJ�����________��
(6)β��Ӧ��δ�����_________��
(7)�����д����ļ���ˮ����������ļ�����Ҵ���ȫȼ�ղ�����������CO2�϶����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
ʵ������ȡ���ᶡ����ʵ��װ����������ͼ��ʾ����װ�ù�ѡ�á����й����ʵ���������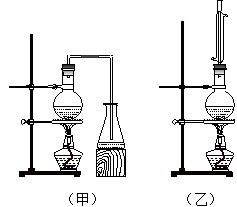
���±���
| | ���� | 1-���� | ���ᶡ�� |
| �۵�(��) | 16.6 | ��89.5 | ��73.5 |
| �е�(��) | 117.9 | 117 | 126.3 |
| �ܶ�(g/cm3) | 1.05 | 0.81 | 0.88 |
| ˮ���� | ���� | ����(9g/100gˮ) | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�� 11�֣��Ҵ��ķе���78�棬����ˮ������Ȼ��ܡ����ѵķе�Ϊ34.6�棬������ˮ���ڱ���Na2CO3��Һ�м������ܣ����Ѽ���ȼ�ա�ʵ�������ѵķ�Ӧԭ���ǣ�
2CH3CH2OH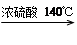 H2O + CH3CH2��O��CH2CH3 (����)
H2O + CH3CH2��O��CH2CH3 (����)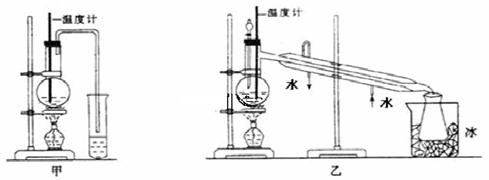
��1����ͼ����ͼ������ʵ���������ѵ�װ�ã�ѡװ��___(��ס����ҡ�)�������������_ ��
��2����ӦҺ��Ӧ�����ʯ����������____________��
��3����Ӧ���¶ȼƵ���ȷλ����ˮ��������________________________��
��4��������װ�����Ƶõ������п��ܺ��д��������ʣ���������__________����ȥ�������ʵļ�������________________________________��
��5������¶�̫�ߣ���170�棩�����ᷢ��һ���л�����Ӧ����Ӧ����ʽΪ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ڡ���ɫ��ѧ�������У������״̬�Ƿ�Ӧ���е�ԭ��ȫ��ת��Ϊ���������ղ����ԭ�ӵ�������Ϊ100%�����з�Ӧ�����������֡�ԭ�Ӿ����ԡ�ԭ����ǣ� ��
���û���Ӧ �ڻ��Ϸ�Ӧ �۷ֽⷴӦ ��ȡ����Ӧ �ݼӳɷ�Ӧ �Ӿ۷�Ӧ
| A���٢ڢ� | B���ڢݢ� | C���ۢ� | D��ֻ�Т� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com