
����Fe(OH)2���ױ�����������ʵ����������������Һ���ռӦ�Ƶð�ɫ������Fe(OH)2������������ͼ��ʾʵ��װ�ÿ��Ƶô�����Fe(OH)2�������������Ϸֱ�Ϊʯī������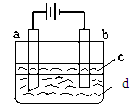
��1��b�缫����Ϊ______����缫��ӦʽΪ_____________________��
��2����ѡ����գ����ʱ�����ڵ缫���а�ɫ��������ʱ�����Һd��_____����������֮�����Һ���а�ɫ��������ʱ�����Һd��______��
| A����ˮ | B��NaCl��Һ | C��NaOH��Һ | D��CuCl2��Һ |
��1��ʯī��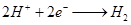
��2��C,B
��3��������������ֹ���ﱻ�������Ͼ���Һ�е�����
��4����ɫ����Ѹ�ٱ�ɻ���ɫ������Ϊ���ɫ��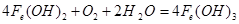
���������������1��b�缫���Ÿ������������������õ����ӱ���ԭ�������DZ������ģ�������ʯī����Һ�е��������������õ����ӱ���ԭ��
��2�����ʱ�����ڵ缫���а�ɫ��������ʱ��������Һ�����������Ӻֻܶ࣬��������������Һ����������֮�����Һ���а�ɫ��������ʱ�������������������ڵ������в����ģ��������ҺӦ���Ȼ��ơ�
��3�������ܶȱ�ˮ���ܶ�С���Ҳ�����ˮ�����Ը�������Һ�ı��棬����Һ�������������ֹ�����е���������Һ���������ã����ȿ��Խ���������ˮ�е��ܽ�ȣ��Ӷ��ﵽ�Ͼ���Һ��������Ŀ�ģ�
��4������Na2SO4��Һ�����Һʱ��ˮ�е������ӱ���ԭ��������������Fe(OH)2������
���㣺�����������֪ʶ�㡣


 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣�п�������ǻ��ý����������������������ǿ�ᣬ��������ǿ������������������ڰ�ˮ��������п���ڰ�ˮ����Zn��NH3��22�����ش��������⣺
��1����������������������Һ����Һ����Ԫ�صĴ�����ʽΪ ���û�ѧʽ����� ��2��д��п������������Һ��Ӧ�Ļ�ѧ����ʽ ��
��3�����и����е�������Һ������μӵ�ʵ�鷽�����ɼ������ ��
������������������ ���������Ͱ�ˮ ������п���������� ������п�Ͱ�ˮ
��4��д�������������백ˮ��Ӧ�����ӷ���ʽ____________________________________________�� �Խ�����ʵ���Ҳ������ÿ�����п���백ˮ��Ӧ�Ʊ�������п��ԭ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������A��B�ͷǽ�������C��D�Լ����ǻ�����֮���ת����ϵ���¡�F��J��������ǿ��M��������ǿ��N��Z��Ħ������Ϊ198 g��mol��1�������и�Ԫ�ص�������Ϊ����:����B:����39:28:32��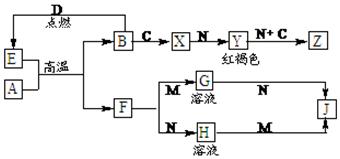
��ش��������⣺
��1��C�Ļ�ѧʽΪ ��Z�Ļ�ѧʽΪ ��
��2��д������X�������ӵķ��� ��
��3��д��E��A�ڸ����·�Ӧ�Ļ�ѧ����ʽΪ ��
��4��д��A��N��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ú���A12O3��SiO2������FeO·xFe2O3�������Ʊ�A12(SO4)3·18H2O��������������(���ֲ�����������)��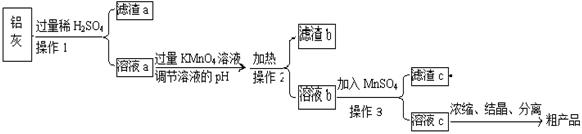
��1������a���������� ����������������������������������
��2���뽫MnO4������Fe2+�����ӷ���ʽ����������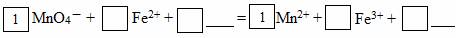
����Ӧ��ת����2mol���ӣ������������������ʵ���Ϊ mol������
��3����֪�����������������pH���£�
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����仯����֮����ת��������ʽ��ʾ��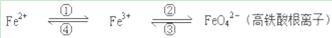
�ش������й����⣺
��1����Ԫ�������ڱ��е�λ����___________________________��
��2��Fe�����ϡ���ᷴӦ������ȡFeSO4�����÷�Ӧ���õ�������Һ��ʵ�������ٵ�ת����Ҫ����﴿������ѡ�õ��Լ���________��ѡ����ţ���
A��Cl2 B��Fe C��HNO3 D��H2O2
��3������ת���õ��������������ڵ绯������ͭ���ա����������������ķ�Ӧ�Ƚϸ��ӣ�������һ��Ҫ��ӦCuFeS2+4Fe3+��Cu2++5Fe2++2S��CuFeS2��SΪ-2�ۣ�������˵����ȷ����________��ѡ����ţ���
A�������ʷ���ĽǶȿ�����ͭ�����ںϽ�
B����Ӧ�У�������Ԫ�ؾ�����ԭ
C����Ӧ�У�CuFeS2����������������ԭ��
D����ת��1 mol����ʱ��46 g CuFeS2�μӷ�Ӧ
��4��������Ӧ�У���FeSO4��O2��ϵ����Ϊ2�U1������ƽ���з���ʽ��
FeSO4 + K2O2 �� K2FeO4 + K2O + K2SO4 + O2��
��5����ijϡHNO3��Fe(NO3)3�Ļ����Һ���������ۣ�������ɫ���壬 �����������������ɫ����Һ��Fe2+Ũ�Ⱥͼ���Fe�۵����ʵ���֮��Ĺ�ϵ����ͼ��ʾ��������Һ��HNO3��Fe(NO3)3�����ʵ���Ũ��֮��Ϊ__________��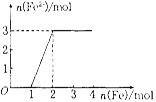
��6��ijͬѧ������ˮ�������·�Ӧ��Ĺ��������ܽ��ڹ��������У�����ж�������Һ���Ƿ���Fe3+�� ___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ִ������ͭ�ڲ�ͬ�����й㷺��Ӧ�á�ijͭ��ʯ������ͭ��������ͭ����������������ʯ��SiO2)���ֲ���������ӿ�ʯ����ȡͭ���乤������ͼ���¡�����ͭ����ȡ��ͭ��ˮ������л���Ĺ��̣��ͷ���ȡ��ͭ���л������ˮ��Ĺ��̣����ִ�ʪ����ͭ����Ҫ�����ֶΡ�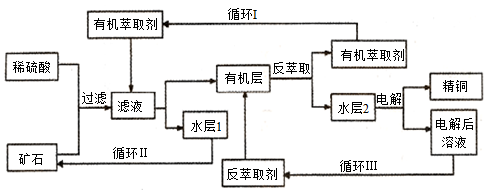
��֪������ȡ���ˮ��2������ͭ��Һ���ش��������⣺
(1����ʯ��ϡ���ᴦ�������з����ಽ��Ӧ�����˷���Cu2O+2H+��Cu2++Cu+H2O��Fe2O3+6H+��2Fe3++3H2O��Ӧ�⣬������Ӧ�����ӷ���ʽΪ____________________________________________��
(2)������Һ���Ƿ���Fe3+�ķ�����____________________________________________________��
(3)��ѭ��I�������ѭ�����ˮ��1���ܼ���ѭ��ʹ�ã����ɷ����һ����Ҫ�������Σ���ˮ��1��¶�ڿ�����һ��ʱ����Եõ���һ����Ҫ�������Σ�д��ˮ��l��¶�ڿ����з�����Ӧ�����ӷ���ʽ________ _____________��
(4��д�������������������Ե缫��������Ӧ�ĵ缫��Ӧʽ __________________��
(5)��ѭ�����з���ȡ������Ҫ�ɷֵĻ�ѧʽ��________________��
(6) ��ͭ����Ҫ�ɷ�CuFeS2������ȡͭ����Ҫԭ�ϣ��ɲ��û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��Cu2S��2Cu2O��6Cu+SO2�����÷�Ӧ�У�_______ (�ѧʽ)����ԭ����ÿ����1mol Cu����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ������Ȼ������Ҫ��������������Ҫ�ɷ�ΪAl2O3��������FeO��SiO2���С���ҵ�����������Ʊ�����ij�ֻ�����Ĺ����������£�
��1����ҺA�������������ε���___________________��
��2��������ҺB���Ƿ�����Ԫ�صķ���Ϊ��_______________________________________
____________________________________________________________��ע���Լ�������
��3������ҺB�е���Ԫ���Գ�����ʽ����������Լ�Ӧѡ_____________������ţ���
a������������Һ b��������Һ c����ˮ d��������̼
��4��SiO2��NaOH�����Ʊ������ƣ��ɲ��õ�װ��Ϊ___________������ţ���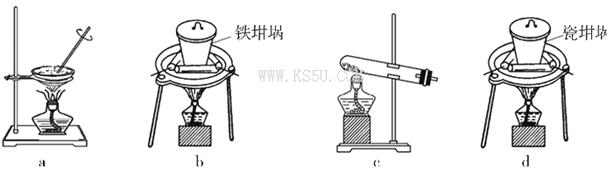
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������Ҫ�ɷ���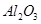 ����
����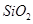 ��
��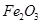 ��MgO�����ʣ�����ȡ���ֹ���Ʒ���������£�
��MgO�����ʣ�����ȡ���ֹ���Ʒ���������£�
��ش��������⣺
��1�������Ҽ����ռ������ӷ���ʽΪ_________________________________________.
��2������A��Ӧ��_________________________________________.�����㣩
��3����ҺD������CO2��Ӧ�����ӷ���ʽΪ__________________________________��
�����ҺK�м�������ʯ��ˮ�����ӷ���ʽ��________
��4�������������������ŵ������õ��Լ��Ͼ��ã�ȱ����__________________________
��5����֪298Kʱ�� ���ܶȻ�����
���ܶȻ�����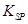 =10-11,ȡ��������ҺB,����һ�������ռ�ǡʹþ���ӳ�����ȫ������Һ��PH��СΪ_______.
=10-11,ȡ��������ҺB,����һ�������ռ�ǡʹþ���ӳ�����ȫ������Һ��PH��СΪ_______.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��15�֣���ͭ�Ͻ�㷺���ں��չ�ҵ�����и�����л��������Ʊ�ͭ������Ʒ�Ĺ������£�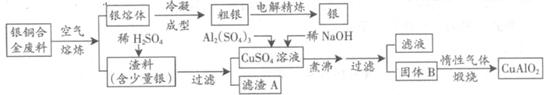
��ע��Al(OH)3��Cu(OH)2��ʼ�ֽ���¶ȷֱ�Ϊ450���80�棩
��1����⾫����ʱ��������ӦʽΪ ������A��ϡHNO3��Ӧ�������������ڿ�����Ѹ�ٱ�Ϊ����ɫ���������ɫ�Ļ�ѧ����ʽΪ ��
��2����������B�����Ϊ �������ɹ���B�Ĺ����У������NaOH�ļ���������NaOH�����������������ķ�Ӧ�����ӷ���ʽΪ ��
��3��������չ�����һ����Ӧ�Ļ�ѧ����ʽ�� CuO+ Al2O3 CuAlO2 + ����
CuAlO2 + ����
��4������ͭ�Ͻ���ͭ����������Ϊ63.5%��������5.0kg�����е�ͭ����ȫת��Ϊ mol CuAlO2��������Ҫ1.0mol?L��1��Al2(SO4)3��Һ L��
��5��CuSO4��ҺҲ�������Ʊ������������������ �����ˡ�ϴ�Ӻ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com