
�����Ϊ2 L�ĺ����ܱ������з�����ӦxA(g)+yB(g) zC(g)��ͼI��ʾ200��ʱ������A��B��C���ʵ�����ʱ��ı仯��ͼ���ʾ��ͬ�¶���ƽ��ʱC�������������ʼn(A):n(B)�ı仯��ϵ�������н�����ȷ����
zC(g)��ͼI��ʾ200��ʱ������A��B��C���ʵ�����ʱ��ı仯��ͼ���ʾ��ͬ�¶���ƽ��ʱC�������������ʼn(A):n(B)�ı仯��ϵ�������н�����ȷ����

A��200��ʱ����Ӧ�ӿ�ʼ��ƽ���ƽ������v(B)= 0. 02 mol��L��1��min��1
B��ͼ����֪��ӦxA(g)+yB(g) zC(g)�ġ�H<0����a=2
zC(g)�ġ�H<0����a=2
C������ͼ����ʾ��ƽ��״̬�£�������ϵ�г���He�����´ﵽƽ��ǰv(��)>v(��)
D��200��ʱ���������г���2 mol A ��1 mol B���ﵽƽ��ʱ��A ���������С��0.5
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �л���Ľṹ���á�����ʽ����ʾ�����磺CH3��CH��CH��CH3�ɼ�дΪ�� ��
�л���Ľṹ���á�����ʽ����ʾ�����磺CH3��CH��CH��CH3�ɼ�дΪ�� ��
 �л���X�ļ���ʽΪ��
�л���X�ļ���ʽΪ��
���л���Y��X��ͬ���칹�壬�����ڷ�������
д��Y�Ľṹ��ʽ��
��X��������H2��һ�������·�Ӧ�����ɻ�״�ı�����Z ��Z��һ�ȴ����� �֡���ṹ��ʽ�ֱ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��KClO3��6HCl(Ũ)===KCl��3Cl2����3H2O.����ͼ��ʾ���������Լ��ֱ�����������е���Ӧλ�ã�ʵ��ʱ��Ũ�������KClO3�����ϣ����ñ�����Ǻã��±�����ʵ������ó��Ľ�����ȫ��ȷ����(����)

| ѡ�� | ʵ������ | ���� |
| A | ����KSCN��FeCl2��Һ��� | Cl2���л�ԭ�� |
| B | ���з�̪��NaOH��Һ��ɫ | Cl2�������� |
| C | ʯ����Һ�ȱ�����ɫ | Cl2����Ư���� |
| D | KI������Һ���� | Cl2���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Һ��һ�������Ե���
A��c(H+)��1��10-7mol/L����Һ
B��pH��pOH ����Һ��pOH��OH��Ũ�ȵĸ�������
C��pH��14��pOH ����Һ
D���ɵ�����������ʵ���Ũ�ȵ�һԪ�������������Һ��Ϻ����γɵ���Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ƕ�Ԫ���ᣬ���������Һ�����ԡ���0.1mol��L��1 KHC2O4��Һ�У����й�ϵ��ȷ����
A��c(K��)��c(H��)��c(HC2O4��)��c(OH��)��c(C2O42��)
B��c(HC2O4��)��c(C2O42��)��0.1mol��L��1
C��c(C2O42��)��c(H2C2O4)
D��c(K��)��c(H2C2O4)��c(HC2O4��)��c(C2O42��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧʵ��С����Ҫ�˽��г�������ʳ�ð״�(��Ҫ�Ǵ����ˮ��Һ)��Ũ��,�ִ��г�������һƿijƷ��ʳ�ð״ף���ʵ���ұ�NaOH��Һ������еζ����±���4�ֳ���ָʾ���ı�ɫ��Χ��
| ָʾ�� | ʯ�� | ���� | ���� | ��̪ |
| ��ɫ��Χ��pH�� | 5.0��8.0 | 3.1��4.4 | 4.4��6.2 | 8.2��10.0 |

��1����ʵ��Ӧѡ�� ��ָʾ��������ƿ����ȡһ������İ״����õ������� ��
��2����ͼ��ʾ50mL,�ζ�����Һ���λ�ã���A��C�̶ȼ����1 mL,A���Ŀ̶�Ϊ25,�ζ�����Һ�����ӦΪ mL��
��3���ڵζ��Ĺ����У�ʱ�������ἷѹ��ʽ�ζ��ܵIJ��������ֱߵα�����ƿ���۾�Ӧ�۲� ���� ���ζ��յ��жϣ���ֹͣ�ζ�����¼�ζ��ܵļ�����
��4��Ϊ�˼�Сʵ�����,��ͬѧһ������������ʵ��,����ÿ����ȡ�״������ΪVmL, NaOH��ҺŨ��Ϊc mol��L-1,����ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.32 | 25.28 |
���ϱ����Կ���,��һ��ʵ���м�¼����NaOH��Һ��������Զ��ں�����,��ԭ������� ��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱNaOH��Һ�����
B���ζ�ǰ�ζ��ܼ���������,�ζ�����������
C��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ��,δ�ñ�Һ��ϴ
D����һ�εζ��õ���ƿ�ô�װҺ��ϴ��,������δ��ϴ
E���μ�NaOH��Һ����,δ�����,�տ�����Һ��ɫ,����ֹͣ�ζ�
��5��������������,д������ð״��д������ʵ���Ũ�ȵı���ʽ���ػ���)�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ�۷���ϡ�����У����Ⱥ�������������������������һ�����ʺ�ͭ�۵��������٣���Һ����ɫ��ͬʱ�������ݳ����������ǣ� ��
A��Al2(SO4)3 B��Na2CO3 C��KNO3 D��FeSO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£����и���������ָ����Һ��һ���ܴ����������(����)
A�������̪��Һ�Ժ�ɫ����Һ�У�K����Na����Cu2����SO
B���������NaOH��Һ������ϡH2SO4ʱ�����ܲ�����ɫ��������Һ��K����Ba2����Cl����HCO
C��ˮ���������c(H��)��10��13 mol·L��1����Һ�У�Na����Cl����NO ��CH3COO-
��CH3COO-
D��pH=1����Һ�У�Na����NO ��S2-��Cl��
��S2-��Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Խ�����Ʒ���п���ʴ���������ӳ���ʹ��������
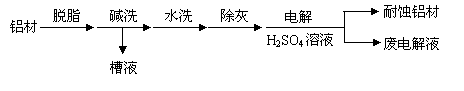 ��1������Ϊ���ı��洦����һ�ַ�����
��1������Ϊ���ı��洦����һ�ַ�����
�� ��ϴ��Ŀ���dz�ȥ���ı������Ȼ����Ĥ��Ϊ����ϴ��Һ�����Գ�����ʽ���գ�������Һ�м��������Լ��е� ��
a��NH3 b��CO2 c��NaOH d��HNO3
��������Ϊ��������H2SO4 ��Һ�е�⣬���ı����γ�����Ĥ�������缫��ӦΪ
��ȡ�����ϵ��Һ������NaHCO3����Һ��������ݺͰ�ɫ����������������ԭ���� ��
��2����ͭ�ɷ�ֹ����Ʒ��ʴ�����ʱ��ͭ������ʯī��������ԭ���� ��
��3��������ͼװ�ã�����ģ�����ĵ绯ѧ������
��XΪ̼����Ϊ�������ĸ�ʴ������KӦ���� ������XΪп������K���� ����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com