
����Ŀ�����ڱ�ǰ�����ڵ�Ԫ�� A��B��C��D��E��ԭ��������������A�ĺ����������������������ͬ��B��Dλ��ͬһ������δ�ɶԵ���������������������EΪ��������Ԫ�أ������ֻ��һ�����ӣ����������й�����������ӡ�
(1)B��C��D����Ԫ�ص�һ�������ɴ�С��˳��Ϊ___(��Ԫ�ط���)��E��̬ԭ�Ӽ۲�����Ų�ͼΪ_____��
(2)д��������Ԫ����ɵ�BD2�ĵȵ�����ķ��� _________��
(3)��֪D���γ�D3+���ӣ�����������ԭ���ӻ���ʽΪ___�����幹��Ϊ__��
(4)�¶Ƚӽ��е�ʱ��D�ļ��⻯���ʵ����������Ը�����ԭ�����ͻ�ѧʽ��������ķ�������ԭ���� _______��
(5)��ɫ��[E(CA3)2]+�ڿ����в��ȶ�������������������ɫ��[E (CA3)4]2+������������ʿɳ�ȥ�����е��������÷�Ӧ�����ӷ���Ϊ________��
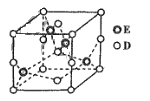
(6)��֪E��D�γɵ�һ�־�����ṹ��ͼ��ʾ����֪�����߳�Ϊanm�������ӵ�����ΪNA����þ�����ܶ�Ϊ_________ g/cm3(�г��������ʽ����)��
���𰸡�N��O��C ![]() N2O sp2 V�� ˮ�����дֵ�ˮ������Ϊ�������ϣ��γɵϷ��� 4[Cu(NH3)2]++O2+8NH3H2O=4[Cu(NH3)4]2++4OH-+6H2O
N2O sp2 V�� ˮ�����дֵ�ˮ������Ϊ�������ϣ��γɵϷ��� 4[Cu(NH3)2]++O2+8NH3H2O=4[Cu(NH3)4]2++4OH-+6H2O ![]()
��������
A�ĺ����������������������ͬ����A��HԪ�أ�B��Dλ��ͬһ������δ�ɶԵ��������������������������߶�Ϊ�������ڣ�δ�ɶԵ�����Ϊ3������������Ԫ�ص�������ֻ��һ�������������⣬��B��DΪ�ڶ����ڣ��������2��δ�ɶԵ��ӣ���2p2��2p4������BΪCԪ�أ�DΪOԪ�أ���CΪNԪ�أ�EΪ��������Ԫ�أ������ֻ��һ�����ӣ����������й�����������ӣ���E��CuԪ�ء�
(1)ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ�������Ԫ�أ���C��N��O�ĵ�һ�����ܴ�С��ϵΪ��N��O��C��CuΪ29��ԭ�ӣ���������Ų�ʽΪ[Ar]3d104s1����۵����Ų�ͼΪ![]() ��
��
(2)BD2ΪCO2������3��ԭ�ӣ��۵�����Ϊ16���ȵ�������ָԭ��������ȣ��۵���������ȵ���������������Ԫ����ɵ���CO2��Ϊ�ȵ�����ķ���ΪN2O��
(3)DΪOԪ�أ�����D3+����ΪO3+��������ԭ�ӵļ۲���Ӷ���Ϊ2+![]() =2.5������3���㣬����Ϊsp2�ӻ����µ��Ӷ���Ϊ1���������幹��ΪV�Σ�
=2.5������3���㣬����Ϊsp2�ӻ����µ��Ӷ���Ϊ1���������幹��ΪV�Σ�
(4)�¶Ƚӽ�ˮ�ķе��ˮ�����д��ڴֵ�ˮ������Ϊ�������ϣ��γɵϷ��ӣ�������ⶨֵƫ��
(5)��ɫ��[Cu(NH3)2]+�ڿ����в��ȶ�������������Ϊ����ɫ��[Cu(NH3)4]2+�����������������÷�ӦӦ�ڰ�ˮ�н��У����Ԫ���غ��֪�ù����л�����������ˮ���ɣ����ӷ���ʽΪ4[Cu(NH3)2]++O2+8NH3H2O=4[Cu(NH3)4]2++4OH-+6H2O��
(6))Cu��O�γ�һ�־��壬�þ�����Cuԭ�Ӹ���=4��Oԭ�Ӹ���=8��![]() +6��
+6��![]() =4�����Ծ���������Ϊ
=4�����Ծ���������Ϊ![]() g���þ������V=(a��10-7 cm)3����þ����ܶ�
g���þ������V=(a��10-7 cm)3����þ����ܶ�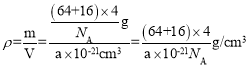 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڹ�ҵ����������β���к��ж��ֵ��������Ҫ��NO ��NO2������NOx����ʾ��NOx���ƻ������㣬�����⻯ѧ����������ɴ�����Ⱦ����Ҫ��Դ֮һ���ش��������⣺
(1)��֪ 1mol ���ӷֽ�Ϊ����ԭ������Ҫ������Ϊ�����ʡ� N2(g)��NO(g)��O2(g)�Ľ����ʷֱ�Ϊ941.7��631.8��493.7(��λkJ/mol)�����㷴Ӧ 2NO(g) = N2(g) + O2(g)����H=_______kJ/mol�����ж�NO(g)���¡���ѹ���ܷ��Է��ֽ� ________���ܻ���)��
(2)Ϊ��ֹ�⻯ѧ���������ӹ�������������ƽ��иĽ��⣬ҲҪ����ijЩ��ѧ�������ý�̿��ԭNOx �ķ�ӦΪ2NOx(g) + xC(s)N2(g) + xCO2(g)
��.�ں��������£�2 molNO2(g)������ C(s)��Ӧ�����ƽ��ʱ NO2(g)�� CO2(g)�����ʵ���Ũ����ƽ����ѹ�Ĺ�ϵ��ͼ��ʾ��
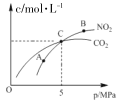
��A��B����NO2ƽ��ת���ʵĹ�ϵ��(A)____��(B)��ƽ�ⳣ����ϵK(A)_____K(B)(�������)��
�ڼ���C��ʱ�÷�Ӧ��ѹǿƽ�ⳣ��Kp=_____MPa(Kp����ƽ���ѹ����ƽ��Ũ��)���㣬��ѹ=��ѹ�����ʵ�������)��
��.�������ݻ���Ϊ 2L �������ܱ�����A��B�м���һ������ NO(g)��������C(s)����ͬ�¶��²����������n(NO)��ʱ��仯��������ʾ��
0 | 20 | 40 | 60 | 80 | |
n(NO)/mol(A) | 2 | 1.5 | 1.1 | 0.8 | 0.8 |
n(NO)/mol(B) | 1 | 0.8 | 0.65 | 0.53 | 0.45 |
B�����ڷ�Ӧ�� 100sʱ�ﵽƽ��״̬����0~100s���� NO ��ʾ��ƽ����Ӧ����Ϊv(NO)= ____________��
(3)�������绯ѧ�����ڴ����������﷽��Ҳ����һ�������ã���ͼ��һ�ְ���һ��������ȼ�ϵ�أ������¿ɽ���������ת��Ϊ������

��c��ͨ�������Ϊ______ ��д��������Ӧ�ķ���ʽ ________��
����a��d�ڲ��������������Ϊ1.568L(�����)����·��ͨ���ĵ�����Ϊ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����Ȼ�ѧ����ʽ��2Zn(s) + O2(g) ��2ZnO(s) ��H1����702.2kJ/mol
Hg(l) + ![]() O2(g) ��HgO(s) ��H2����90.7kJ/mol
O2(g) ��HgO(s) ��H2����90.7kJ/mol
�ɴ˿�֪Zn(s) + HgO(s) �� ZnO(s) + Hg(l)����H3 ��������H3��ֵ���� ��
A. ��260.4 kJ/mol B. ��254.6 kJ/mol
C. ��438.9 kJ/mol D. ��441.8 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����и��������У���Ϊͬλ�ص���_____������ţ���ͬ������Ϊͬϵ�����_____����Ϊͬ���칹�����_____��
�ٺ�������� ��35Cl��37Cl ��CH3COOCH3��CH3CH2COOCH3��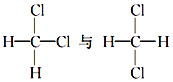 ��
��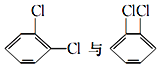 ��
��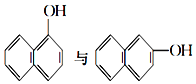 ���Ҵ��������
���Ҵ��������
��2����ϵͳ�������������л���������
��CH3CH��C2H5��CH��CH3��2��_____��
��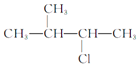 _____
_____
��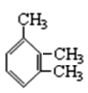 _____
_____
��3��д�������л���Ӧ�ķ���ʽ��
��1,3-����ϩ�ļӾ۷�Ӧ��_____
��3-��-2-�����Ĵ�������Ӧ��_____
�ۼ�ȩ������������ͭ��Ӧ��_____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ���װ�ý������е�SO2��NOת��Ϊ(NH4)2SO4��������Ϊһ�������ĵ��ʡ������й�˵����ȷ����
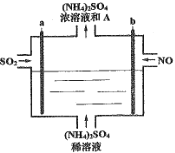
A.a���Դ����������������ԭ��Ӧ
B.ÿ����lmolNO��������2molA
C.ͨ�������������Һ��pH����
D.�����Ͻ�SO2��NO�������2:5ͨ��װ�ÿɳ���ת��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������� (Na2S2O3)�����������о��й㷺Ӧ�á���ǹ�ҵ����ȡ��������Ƶķ���֮һ��ʵ����ģ�ҵ����װ����ͼ��ʾ��
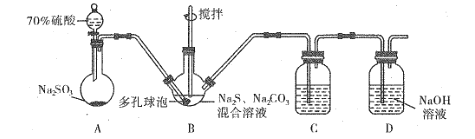
(1)������ͼװ�ý���ʵ�飬Ϊ��֤����˳�����µIJ�����_______��
(2)װ��B�����ɵ�Na2S2O3ͬʱ������CO2����Ӧ�����ӷ���ʽΪ_______���ڸ�װ����ʹ�ö�����ݵ�Ŀ����_____��
(3)װ��C�������Ǽ���װ��B��SO2������Ч����C�п�ѡ����Լ���__(����ĸ)��
a.H2O2��Һ b.��ˮ c.KMnO4��Һ d.BaCl2��Һ
(4)Na2S2O3��Һ�����ڲⶨ��ˮ��Ba2+Ũ�ȡ�
��ȡ��ˮ20.00mL�������ʵ�����ȣ���������K2Cr2O7��Һ���õ� BaCrO4 ����������ϴ�Ӻ�������ϡ���ܽ⣬��ʱ CrO42-ȫ��ת��ΪCr2O72-���ټӹ��� KI��Һ����Cr2O72- ��ַ�Ӧ��Ȼ����������Һ��ָʾ������0.100 mol/L��Na2S2O3 ��Һ���еζ���(I2 +2 S2O32-= S4O62-+ 2I-)���ζ��յ������Ϊ__________��ƽ�еζ�3�Σ�����Na2S2O3 ��Һ��ƽ������Ϊ18.00mL����÷�ˮ��Ba2+ �����ʵ���Ũ��Ϊ____mol/L��
���ڵζ������У�����ʵ����������ʵ����ƫ�ߵ���______(����ĸ)��
a.�ζ���δ��Na2S2O3��Һ��ϴ
b.�ζ��յ�ʱ���Ӷ���
c.��ƿ������ˮϴ�Ӻ�δ���и��ﴦ��
d.�ζ��ܼ��촦�ζ�ǰ�����ݣ��ζ��յ㷢��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ��Ũ�ȹ�ϵ��ȷ����(����)
A. С�մ���Һ�У�c(Na��)��c(H��)��c(HCO)��c(CO![]() )��c(OH��)
)��c(OH��)
B. CH3COONa��Һ�У�c(CH3COO��)>c(Na��)
C. ���ʵ���Ũ����ȵ�CH3COOH��Һ��CH3COONa��Һ�������ϣ�c(CH3COO��)��2c(OH��)��2c(H��)��c(CH3COOH)
D. 0.1 mol/L��NaHA��Һ����pH��4����c(HA��)>c(H��)>c(H2A)>c(A2��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ���ǣ� ��
A.T��ʱ��pH=7����Һһ��������
B.��֪��Ӧ2NO2(g)![]() N2O4(g)�ڵ����¿��Է����У�����H>0
N2O4(g)�ڵ����¿��Է����У�����H>0
C.��0.1molL-1��NH4Cl��Һ�У�c(NH4+)+c(NH3H2O)=0.1molL-1
D.��Na2CO3��Һ�У�c(Na+)=2c(CO32-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ҫ��Ԫ�أ����γɶ��ֺ����ᡣ�ش���������:
(1)������(H3PO2)��һԪ�ᣬ����볣����ֵK=9��103����10mL0.1 molL-1H3PO2��Һ�м���30mL�����ʵ���Ũ�ȵ�NaOH��Һ��д����Ӧ�����ӷ���ʽ_________��c(Na+)+(H2PO2-)+c(H3PO2)=______(���Ի�Ϻ��ܲ�����ı仯)��
(2)������(H3PO3)�Ƕ�Ԫ���ᣬ 25��ʱ������ĵ��볣����ֵΪK1=1��10-2��k2=2.6��10��7,��NaH2PO3��Һ����_____(��ᡱ������С�)��ԭ����____(��ϻ�ѧ���P���ݼ�����н���)
(3)��֪HF�ĵ��볣����ֵΪK=3.6��10-4,������HF��Һ��Na2HPO3��Һ��Ӧ�������ӷ���ʽΪ______��
(4)���������ǿ��ԭ�ԡ���ѧʵ��С�����õζ����ⶨij��������Һ��Ũ�ȣ�ȡ25.00mL����������Һ������ƿ�У���0.10 molL-1�ĸ��������Һ���еζ�����Ӧ�����ӷ���ʽ��5H3PO3+ 2MnO4-+6H+ = 5H3PO4+ 2Mn2+ +3H2O��
���εζ�ʵ������ݷֱ�����:
ʵ���� | �ζ�ǰ���� | �ζ������ |
1 | 0.50 | 22.50 |
2 | 1.50 | 24.50 |
3 | 1.00 | 22.00 |
����������Һ�����ʵ���Ũ��Ϊ______��
�ڹ��ڸ�ʵ������˵����ȷ����______(��д���)��
a ȡ��������Һ�ĵζ��ܣ�ϴ�Ӻ�δ��ϴ�����½��ƫ��
b ʢ���������Һ�ĵζ��ܵζ�ǰ�����ݣ��ζ��������ݣ����½��ƫ��
c �ζ��������۾�ֻע�ӵζ�����Һ��仯�������ü�¼
d ��ƿδ����ײ���ˮ���ᵼ�½��ƫ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com