
 ��ͻȻ���ҷ�Ӧ�����������ݣ��ų�����ɫ���壬����Һ��ɫ��ʧ��Ϊdz��ɫ����Һ�¶����ߣ���������KSCN��Һ��Ϊdz��ɫ������Ϊ��ɫ��������������ͨ�������Ba(OH)2��Һ���������ǣ���ʣ��һ�ַǼ������壻��Ӧ�����Һ�м���BaCl2��Һ������ɫ������(�˹�������Һ��ɫ�仯����ϸ��)����д����ŨHNO3�е���KSCN���ӵķ���ʽ�� ��
��ͻȻ���ҷ�Ӧ�����������ݣ��ų�����ɫ���壬����Һ��ɫ��ʧ��Ϊdz��ɫ����Һ�¶����ߣ���������KSCN��Һ��Ϊdz��ɫ������Ϊ��ɫ��������������ͨ�������Ba(OH)2��Һ���������ǣ���ʣ��һ�ַǼ������壻��Ӧ�����Һ�м���BaCl2��Һ������ɫ������(�˹�������Һ��ɫ�仯����ϸ��)����д����ŨHNO3�е���KSCN���ӵķ���ʽ�� �� ����5��2���ϵ�д�
����5��2���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 He���ֲ��������̽�⡣
He���ֲ��������̽�⡣ He��ʾ�ĺ�ԭ��( )
He��ʾ�ĺ�ԭ��( )| A�����������Ϊ3 | B��������Ϊ3 |
| C������������Ϊ3 | D������������Ϊ3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO2����ˮ����HNO3������NO2��HNO3������ |
| B�������������Բ������ᣬ����������Σ�� |
| C����Ԫ���ǻ��õķǽ���Ԫ�أ����Ե���Ҳ�ܻ��� |
| D����ҵ�����÷���Һ̬�����ķ�����ȡ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ԭ����ȫ����NO����Ӧ�����Һ�м���һ������NaOH��Һ��ǡ��ʹ��Ԫ����ȫת��Ϊ���������ˣ�������ϴ�ӡ�������ա����أ��ù���a�ˣ�����Һ���ɡ����ա����أ��ù���b�ˡ�
ԭ����ȫ����NO����Ӧ�����Һ�м���һ������NaOH��Һ��ǡ��ʹ��Ԫ����ȫת��Ϊ���������ˣ�������ϴ�ӡ�������ա����أ��ù���a�ˣ�����Һ���ɡ����ա����أ��ù���b�ˡ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���� | B���� | C���� | D��п |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��40��32L | B��30.24L | C��20��16L | D��6��72L |
 �����У���˵��ԭ�������У���д����Ӧ�����ӷ���ʽ�� ��
�����У���˵��ԭ�������У���д����Ӧ�����ӷ���ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

 �Ļ�ѧ����ʽΪ ��
�Ļ�ѧ����ʽΪ �� ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ��
ˮCuSO4��ĩ��Ϊ��ɫ��ͬʱ����һ����ɫ���壬����������Ⱦ�� Ӧ�Ļ�ѧ����ʽ
Ӧ�Ļ�ѧ����ʽ  ��
�� 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��N2 | B��NO2 | C��NO | D��N2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
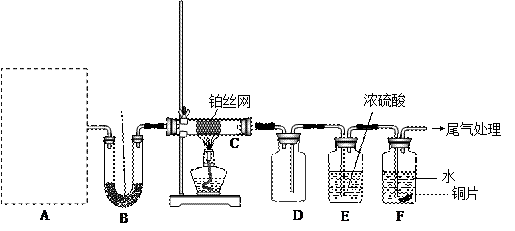
 ���װ�������Ժ��Ƚ�C����˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ơ�����ʵ���������£���˿�������ֺ��ȣ�F��ͭƬ���ܽ⡣
���װ�������Ժ��Ƚ�C����˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ơ�����ʵ���������£���˿�������ֺ��ȣ�F��ͭƬ���ܽ⡣�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com