
| 0.448L |
| 22.4L/mol |
| 2.33g |
| 233g/mol |
| 4.30g-2.33g |
| 197g/mol |
| 0.448L |
| 22.4L/mol |
| 2.33g |
| 233g/mol |
| 4.30g-2.33g |
| 197g/mol |
| 0.01mol |
| 0.1L |
| 0.01mol |
| 0.1L |
| 0.02mol |
| 0.1L |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ca2++2OH-�TCa��OH��2�� CaCO3+2NaOH�TCa��OH��2��+Na2CO3 |
| B��CO32++2H+�TCO2��+H2O BaCO3+2HCl�TBaCl2+CO2��+H2O |
| C��Ca2++CO32-�TCaCO3�� Ca ��NO3��2+NaCO3�TCaCO3+2NaNO3 |
| D��H++OH-�TH2O 2KOH+H2SO4�TK2SO4+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
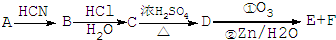
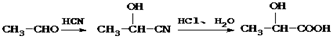
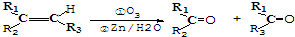
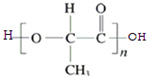 ���ĺϳ�·������ͼ�����Լ���ѡ�ã���
���ĺϳ�·������ͼ�����Լ���ѡ�ã���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | �����Ϣ |
| X | Xԭ�Ӻ����������������ڲ��������2�� |
| Y | Y��Xͬ���ڣ����̬ԭ��ռ��s����ĵ�������ռ��p����ĵ�������ͬ |
| Z | Z��X��ͬ������Ԫ�� |
| W | ԭ������Ϊ29 |
| Q | Q��Zͬ���ڣ����ڸ���������Ԫ����ԭ�Ӱ뾶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
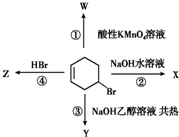 ��ͼ��ʾ4-�廷��ϩ��������4����ͬ��Ӧ��
��ͼ��ʾ4-�廷��ϩ��������4����ͬ��Ӧ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com