
����Ŀ��ijУ��ѧ��ȤС��ͬѧ��������ˮ�п��ܺ��д���Ca2����Mg2����ijЩ�����ӣ��Ӷ�����������ʵ�飺
��ȡ��������ˮ���Թ��У��μ�������NaOH��Һ��������ɫ������
�ڹ��˺�ȡ��Һ���Թ��У��μ�������Na2CO3��Һ�����а�ɫ�������ɣ�
����ȡ��������ˮ���Թ��У��μ�����ϡ������ٵμ�AgNO3��Һ��Ҳ������ɫ������
��ش��������⣺
(1) ͨ��ʵ��ɳ���ȷ������ˮ��________(����������������������)����Ca2����Mg2����
(2) ����ˮ�����������ӿ���ȷ����___________����������AgNO3��Һ������Ӧ�����ӷ���ʽΪ________
���𰸡����� Cl-(��������) ![]()
��������
��1�����ݢ١��ڵ��������ɵİ�ɫ������̼��ƺ�������þ������ȷ������ˮ�к���Ca2+��Mg2+�����ӣ���Ӧ�����ӷ���ʽ�ǣ�CO32-+Ca2+=CaCO3����Mg2++2OH-=Mg(OH)2�����ʴ�Ϊ���У�
��2��Cl-+Ag+=AgCl������ɫ����������HNO3���μ�����ϡ������ٵμ�AgNO3��Һ��Ҳ������ɫ������˵������ˮ�к���Cl-���ʴ�ΪCl-(��������)��Cl-+Ag+=AgCl����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��1��2-����������������������Ӽ�����ͼΪʵ�����Ʊ�1��2-���������װ�Dͼ�� ͼ�з�Һ�ƶ�����ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ���dװ�D�Թ���װ��Һ�塣

��֪��CH3CH2OH![]() CH2=CH2��+H2O��2CH3CH2OH
CH2=CH2��+H2O��2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
��������б����£�
�Ҵ� | 1��2-�������� | ���� | �� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
�ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
�е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
�۵�/�� | -114.3 | 9.79 | - 116.2 | -7.2 |
ˮ���� | ���� | ���� | �� | ���� |
��1��ʵ����ӦѸ�ٽ��¶����l��170�����ҵ�ԭ����______________________________��
��2����ȫƿb��ʵ�����ж������á���һ���Լ��ʵ�������dװ�D�е����Ƿ���������
��д����������ʱƿb�е�����_______________________________�����ʵ��ʱdװ�D�е��ܶ���������Ϊ���ܵ�ԭ���Ǣ�_______________________________________________����ȫƿb�������������Ǣ�__________________��
��3������c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������_______________________________��
��4��ijѧ��������ʵ��ʱ��ʹ��һ������Һ�壬����ȫ����ɫʱ���������Ҵ���Ũ������Һ����������ȷ����³������࣬���װ�D��������û�����⣬�Է������ܵ�ԭ��______________��______________��д���������ɣ���
��5����ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ___________��Ҫ��һ���ᴿ�����в����б������_____________ ������ĸ����
A���ؽᾧ B������ C����ȡ D������
��6��ʵ����Ҳ���Գ�ȥdװ�D��ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�D���Թ��У��� ��ʱ��ˮ����������ȴ1��2-��������������⣬��������������____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪Ǧ���طŵ�ʱ��ط�ӦΪPbO2��Pb��4H����![]() =2PbSO4��2H2O����ͼ��Ǧ���صĹ���ԭ��ʾ��ͼ������˵����ȷ����
=2PbSO4��2H2O����ͼ��Ǧ���صĹ���ԭ��ʾ��ͼ������˵����ȷ����
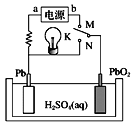
A.K��N����ʱ����װ���е���ת��Ϊ��ѧ��
B.K��N����ʱ��H+���ƶ�
C.K��M����ʱ��aΪ��Դ�ĸ���
D.K��M����ʱ������������Һ��pH������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
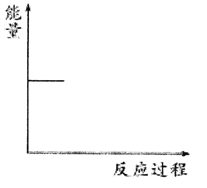
����Ŀ��ij���᳧����β����NO�ķ������ڴ��������£���H2��NO��ԭΪN2�����Ȼ�ѧ����ʽΪNO(g)��H2(g)=![]() N2(g)��H2O(g) ��H��mkJ��mol��1���������仯������ͼ��
N2(g)��H2O(g) ��H��mkJ��mol��1���������仯������ͼ��
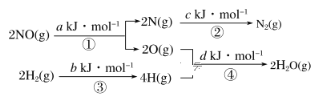
����˵����ȷ���ǣ� ��
A.���̢١��ڡ��ۡ��ܶ��Ƿ��ȹ���B.m����![]() (a��b��c��d)kJ��mol��1
(a��b��c��d)kJ��mol��1
C.m����![]() (c��a��d��b)kJ��mol��1D.m����
(c��a��d��b)kJ��mol��1D.m����![]() (c��d��a��b) kJ��mol��1
(c��d��a��b) kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʵ������������װ�á�
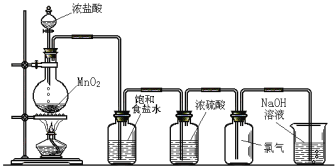
Բ����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ_____________________������1��Cl2����ת�Ƶ�����ĿΪ________��������ʳ��ˮ��������_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�������������������������ɵ��γ�Ϊ���Ρ������Na4S2O3�м�������ϡ���ᣬ������Ӧ��![]() ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��
A. Na4S2O3��ˮ��Һ�Լ���
B.1mol Na4S2O3�й���������Ϊ5NA
C.������Ӧ�У�ÿ����3molS��ת�Ƶ��ӵ����ʵ���Ϊ6mol
D.CaOCl2Ҳ�ɳ�Ϊ���Σ���CaOCl2�м�������ϡ�������Cl2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������̼����������Ϊ��ѧ���о�����Ҫ���⡣��ȼú�����е�CO2ת��Ϊ�����ѵķ�Ӧԭ��Ϊ:
2CO2(g) + 6H2(g) ![]() CH3OCH3(g) + 3H2O(g)
CH3OCH3(g) + 3H2O(g)
(1) ��֪һ�������£��÷�Ӧ��CO2��ƽ��ת�������¶ȡ�Ͷ�ϱ�[n(H2)/n(CO2)]�ı仯��������ͼ:
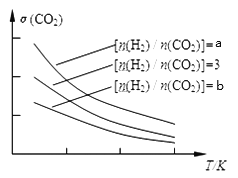
��a��3��b�Ĵ�С��ϵ___________
���������ͼ����Ϣ����ͼ(��)�л���CO2(g)��H2(g)ת��ΪCH3OCH3(g)��H2O(g)��������ϵ����___________��
(2)ij�¶��£���2.0molCO2(g)��6.0molH2(g)�����ݻ�Ϊ2L���ܱ������У�������Ӧ����ƽ��ʱ���ı�ѹǿ���¶ȣ�ƽ����ϵ��CH3OCH3(g)�����ʵ��������仯�����ͼ��ʾ�������¶Ⱥ�ѹǿ�Ĺ�ϵ�ж���ȷ��______
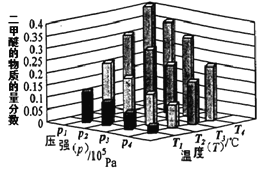
A. P3��P2��T3��T2 B. P1��P3��T1��T3 C. P2��P4��T4��T2 D. P1��P4��T2��T3
(3)�ں����ܱ������ﰴ�����Ϊ1:3����CO2(g)��H2(g),һ��������������Ӧ�ﵽƽ��״̬�����ı䷴Ӧ��ijһ�����������б仯��˵��ƽ��һ�����淴Ӧ�����ƶ�����___________
A. ����Ӧ������������С B. �淴Ӧ������������С
C. ��ѧƽ�ⳣ��Kֵ���� D. ��Ӧ���Ũ������
(4)����һ����˵��������Ӧ�ﵽƽ�����___________
A.�����ƽ��Ħ����������
B.���������£�������ܶȲ���
C.�����ʵ�����֮�ȵ���ϵ����
D.[n(H2)/n(CO2)]����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����ȼ�ϵ�ع���ʾ��ͼ���£����ڸõ�ص�˵������ȷ����

A. �������Һ��Na+��b���ƶ�
B. b���ĵ缫��Ӧ�ǣ�O2+2H2O+4e-=4OH-
C. a���Ǹ���������������Ӧ
D. ����ͨ�����·��b�缫����a�缫
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ͼ��ʾ������������ͨ��ʢ�и�����ɫ�����Ĺ��ƿ��ʢ��ʪ����ɫ�����Ĺ��ƿ���ɹ۲쵽��������_______��
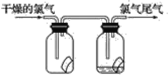
��2��Ϊ��ֹ����β����Ⱦ������ʵ����ͨ����________��Һ���ն����������ԭ����______________________(�û�ѧ����ʽ��ʾ)����ҵ�ϳ������۵�ʯ�������չ�ҵ����β���Ƶ�Ư�ۣ�Ư�۵���Ч�ɷ���________________(�ѧʽ)��Ư������ˮ���ܿ����е�CO2���ã�������Ư�ס�ɱ�����õĴ����ᣬ��ѧ����ʽΪ_____________________������¶���ڿ����е�Ư�ۣ���ϡ����������������________(����ĸ����ͬ)��
A��O2B��Cl2 C��CO2D��HClO
��3��һ����������й©�ͱ�ը�¹ʣ���ΧȺ��Ӧ������ɢ�����������뱬ը�ֳ�ʱ�������ý���һ��Ũ��ij����ˮ��Һ��ë����ס���ӣ������˲��õĸ�������________��
A��NaOH B��NaCl
C��KCl D��Na2CO3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com