
ij��ȤС��Ϊ��֤�ճ������õĻ��ͷ�Ϻ���KClO3��MnO2��S�����������ʵ������ͼ��
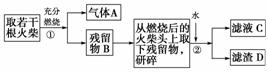
��ش��������⣺
(1)Ϊ��֤����A������ͼ��ʾװ�ý���ʵ�飺���ܹ۲쵽______��������֤�����ͷ�Ϻ���SԪ�ء�
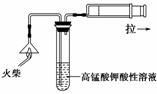
(2)����ڵ�ʵ�����װ����ͼ��ʾ���ò�����������________���乤��ԭ����____________________________��
(3)Ҫ֤�����ͷ�к���ClԪ�صĺ���ʵ�鲽����
_____________________________________________��
(4)��ѧ�����������ͷ��KClO3����һ��ʵ�鷽����

�йص����ӷ���ʽΪ________��
����������������г��ְ�ɫ���������ܳ��˵�����ͷ��KClO3�Ĵ��ڣ���������______________________________________________��
(5)��С��²�����D��˫��ˮ�ֽ������������ʻ����һ����Ӱ�죬��Ʋ�����������5��ʵ�顣
| ʵ����� | H2O2��Һ��������/% | H2O2��Һ����/mL | ����D����/g | ��Ӧ�¶�/�� | �ռ��������/mL | ����ʱ��/s |
| �� | 30 | 5 | 0 | 85 | 2 | 3.8 |
| �� | 15 | 2 | 0.1 | 20 | 2 | 2.8 |
| �� | 15 | 2 | 0.2 | 20 | 2 | 2.2 |
| �� | 5 | 2 | 0.1 | 20 | 2 | 7.4 |
| �� | 30 | 5 | 0 | 55 | 2 | 10.5 |
���ϱ���֪��ʵ��ٺ͢���֤���¶�Խ�ߣ���ѧ��Ӧ����Խ�죬ʵ��________��________��֤������D������Խ��Ӧ����Խ�졣
(6)д��������з�����Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
����������(1)����������AΪ���������������ܱ��������������Һ������(2)�۲�ͼʾ��������������ɣ����Ϊ����ƿ���м�Ϊ��ȫƿ���Ҳ��dz����ã����Ը�װ��Ϊ��ѹ����(�����)װ�á�(3)ȼ�պ������ת��Ϊ�Ȼ��أ���Һ�д��������ӣ���������������Һ���������ӡ�(4)ClO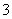 ���������ԣ���NO
���������ԣ���NO ���л�ԭ�ԣ����߷���������ԭ��Ӧ��(5)ʵ��ڡ����¶���ͬ����������D(��������)��������ͬ��ʵ��ۼ����D���ڢڣ������ռ���ͬ���������ʱ��ʵ��������ʱ��̡�(6)����ٷ�����������ѧ��Ӧ��������صķֽ⡢���ȼ�ա������̼��ȼ�յȡ�
���л�ԭ�ԣ����߷���������ԭ��Ӧ��(5)ʵ��ڡ����¶���ͬ����������D(��������)��������ͬ��ʵ��ۼ����D���ڢڣ������ռ���ͬ���������ʱ��ʵ��������ʱ��̡�(6)����ٷ�����������ѧ��Ӧ��������صķֽ⡢���ȼ�ա������̼��ȼ�յȡ�
���𰸡���(1)KMnO4������Һ��ɫ
(2)��ѹ����(�����)����������ˮ��ͷʱ��װ���ڲ��Ŀ���������ˮ�����ߣ�����װ���ڲ�ѹǿ��С��ʹ�����ٶȼӿ죬�õ��ϸ���Ĺ�������
(3)ȡ��ҺC������ϡ�����AgNO3��Һ�����۲쵽�а�ɫ��������������֤�����ͷ�к�����Ԫ��
(4)ClO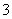 ��3NO
��3NO ��Ag��AgCl����3NO
��Ag��AgCl����3NO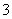
AgNO2��AgCl��Ϊ������ˮ�İ�ɫ����
(5)�ڡ��ۡ�(6)2KClO3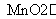 2KCl��3O2����S��O2
2KCl��3O2����S��O2 SO2��C��O2
SO2��C��O2 CO2
CO2


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й����ʵ����ʺ����ʵ�Ӧ�þ���ȷ����
A.������Ũ���������������ۻ������ڳ�������������������Ũ����
B.�������費���κ��ᷴӦ������ʯӢ������������
C.�������Ⱦ��л�ԭ�ԣ�����������ˮ��ɱ������
D.ͭ�Ľ��������Ա����IJ���ں��������װ����ͭ���Լ����丯ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������NOx��NO��NO2�Ļ����(����N2O4)��
(1)���ݷ����ŷű���1 m3�������������400 mg NOx����NOx��NO��������Ϊ0.85����1 m3���������������NO___________________L(��״��������2λС��)��
(2)��ҵ��ͨ����������������Ϊ0.150��Na2CO3ˮ��Һ(�ܶ�1.16 g��mL-1)��ΪNOx���ռ�����̼������Һ���ʵ���Ũ��Ϊ__________________________mol��L-1(����2λС��)��
(3)��֪��NO+NO2+Na2CO3 2NaNO2+CO2��
2NaNO2+CO2��
2NO2+Na2CO3 NaNO2+NaNO3+CO2��
NaNO2+NaNO3+CO2��
1 m3��2 000 mg NOx����������������Ϊ0.150��̼������Һ���ա���������Ϊ80%�����պ������_______________�ŷű�(����ϡ������ϡ�)�����ɣ�_________________��
(4)��������ɸı�������NO��NO2�ıȣ���ӦΪ��NO+2HNO3 3NO2+H2O
3NO2+H2O
��������n(NO)��n(NO2)=2��3ʱ����������ߡ�
1 m3������2 000 mg NOx������n(NO)��n(NO2)=9��1��
���㣺(��)Ϊ�˴ﵽ��������ʣ�1 m3����������������ʵ���(����3λС��)��
(��)1 m3�����ﵽ���������90%ʱ�����պ�����NaNO2������(�����������շ�Ӧ�У���Ӧ�ٱȷ�Ӧ��Ѹ�١�����������1λС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���������0���̶�λ��������ȷ����(����)
A������Ͳ���϶�
B���ڵζ����϶�
C����������ƽ�̶ȳߵ�����
D����������ƽ�̶ȳߵ��ұ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����֪���ʵ���Ũ�ȵ�����ζ�δ֪���ʵ���Ũ�ȵ�NaOH��Һʱ�����в����в���ȷ����(����)
A����ʽ�ζ���������ˮϴ����ֱ�Ӽ�����֪���ʵ���Ũ�ȵ�����
B����ƿ������ˮϴ����ֱ�Ӽ���һ�������δ֪���ʵ���Ũ�ȵ�NaOH��Һ
C���ζ�ǰ��Ҫ�ų��ζ��ܼ��촦������
D������ʱ��������ζ�����Һ�尼Һ����ʹ�����һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʯ��Ҥ��������ʯ�ң�1 mol CaCO3��ȫ�ֽ�����Ҫ����������ȼ��0.453 mol̼���ṩ���������O2�������Ϊ0.21��N2Ϊ0.79����ʯ��Ҥ������������CO2��������������ǣ� ��
A.0.43 B.0.46 C.0.49 D..0.52
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
.Ŀǰ�����ڶ�����̼�Ƿ�Ϊ������Ⱦ���в�ͬ�Ĺ۵㡣��Ϊ��������̼���Ǵ�����Ⱦ���������( )
�ٶ�����̼����Ҫ�Ļ���ԭ��
�ڶ�����̼��ֲ�������õı���ԭ��
�۶�����̼����ɫ����ζ����������
�ܳ�������̼�⣬���顢һ��������Ҳ����������
A.�٢� B.�ڢ� C.�ۢ� D.�٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
U��V��W��X��Y��Z��ԭ������������������ֳ���Ԫ�ء�Y�ĵ�����W2��ȼ�յIJ����ʹƷ����Һ��ɫ��Z��WԪ���γɵĻ�����Z3W4���д��ԡ�U�ĵ�����W2��ȼ�տ�����UW��UW2�������塣X�ĵ�����һ�ֽ������ý�����UW2�о���ȼ�����ɺڡ������ֹ��塣
��ش��������⣺
��1��V�ĵ��ʷ��ӵĽṹʽΪ ��XW�ĵ���ʽΪ ��ZԪ�������ڱ��е�λ���� ��
��2��UԪ���γɵ�ͬ��������ľ������Ϳ����ǣ�����ţ� ��
��ԭ�Ӿ��� �����Ӿ��� �۷��Ӿ��� �ܽ�������
��3��U��V��W�γɵ�10�����⻯���У�U��V���⻯��е�ϵ͵��ǣ�д��ѧʽ��
��V��W���⻯����ӽ��H+������ǿ���ǣ�д��ѧʽ�� ����һ�����ӷ���ʽ����֤�� ��
��4��YW2����ͨ��BaCl2��HNO3�Ļ����Һ�����ɰ�ɫ��������ɫ����VW���йط�Ӧ�����ӷ���ʽΪ ���ɴ˿�֪VW��YW2��ԭ�Խ�ǿ���ǣ�д��ѧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
.��֪��4NH3+5O2 4NO+6H2O 4NO+3O2+2H2O��4HNO3
4NO+6H2O 4NO+3O2+2H2O��4HNO3
��������������������Ϊ0.20�������������Ϊ0.80�������������ռ�����
(1)a mol NO��ȫת��ΪHNO3��Ҫ����_______ mol
(2)ΪʹNH3ǡ����ȫ����Ϊһ����������������������а����������Ϊ________(����2λС��)��
(3)20.0 moL��NH3�ÿ����������������������Ϊ��NO 18.0 mol��O2 12.0 mol��N2 150.0 mol��һ���������ᣬ�Լ������ɷ֡�(������NO��O2����Ӧ)���㰱ת��ΪNO��HNO3��ת���ʡ�
(4)20.0 moL ��NH3��һ����������ַ�Ӧ����ת��ΪHNO3
������ͼ�л���HNO3�����ʵ���n(A)�Ϳ��������ʵ���n(B)��ϵ���������ߡ�
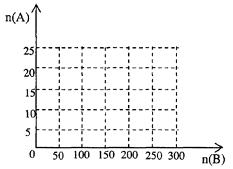
��д����125��n(B)��200ʱ��n(A)��n(B)�Ĺ�ϵʽ______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com