
����Ŀ����84����Һ������Чɱ�����HIN1�Ȳ�����ijͬѧ������һƿ����¶ʿ���ơ�84����Һ����������������Ϻ�����Һ��װ˵���õ�������Ϣ����84����Һ������25%NaClO��1000 mL���ܶ�1.192g/cm3��ϡ��100��������ȣ���ʹ�á������������Ϣ�����֪ʶ�ش��������⣺
��1��100gij84����Һ��3.55g���������������൱���ò�Ʒ����Ч�Ⱦ���3.55%������100gij84����Һ�к���___gNaClO��
��2��һƿ����¶ʿ���ơ�84����Һ����������տ�����CO2___L����״���������ʡ�
��3����ͬѧ���ġ���¶ʿ���ơ�84����Һ�����䷽������NaClO��������480 mL��25%NaClO������Һ������˵����ȷ����___�����ţ���
A.��ͼ��ʾ�������У��������Dz���Ҫ�ģ��������ֲ�������
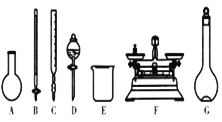
B.����ƿ������ˮϴ����Ӧ��ɲ���������Һ����
C.���ù������ƷNaClO�����ƿ��ܵ��½��ƫ��
D.��Ҫ������NaClO��������Ϊ143 g
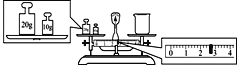
��4��ijͬѧ��������ƽ�����ձ�����������ƽƽ����״̬��ͼ����ͼ�п��Կ������ձ���ʵ������Ϊ___g��
��5������һ�����ʵ���Ũ�ȵ�������Һ�����в������������Ƶ�ϡ�������ʵ���Ũ��ƫ�͵���___(����ĸ)��
A��δ�ָ������¾ͽ���Һע������ƿ�����ж���
B������Ͳ��ȡŨ����ʱ���Ӱ�Һ��
C������ƿ������ˮϴ��δ����
D��δϴ���ձ��Ͳ�����
E������ʱ����Һ��
���𰸡�3.725g 89.6 AC 27.4 BDE
��������
(1). ����Ч�Ⱥ��������������������������������������䶨����:ÿ�˺��������������������൱�ڶ��ٿ�Cl2������������100gij84����Һ��3.55g���������������൱����������Ƶ����ʵ�����������ͬ����m=![]() �õ����ʵ�����
�õ����ʵ�����
��2��c=![]() ����Ũ��������ʵ���Ũ�ȣ����û�ѧ����ʽ�����������̼�����ʵ�����V=n��22.4�����������̼�������
����Ũ��������ʵ���Ũ�ȣ����û�ѧ����ʽ�����������̼�����ʵ�����V=n��22.4�����������̼�������
��3������������Һ�еIJ���������⣻��������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��ʹ�õ�����������c=![]() ��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��4����ƽ��������ʱ��ѭ���������ԭ����ƽƽ��ԭ����������������=������������+����������
��5������һ�����ʵ���Ũ�ȵ���Һ�����Ʋ���ѡ��ʹ�õ�����������c=![]() ��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
��ͨ���жϲ������������ʵ����ʵ���n����Һ���V��Ӱ����������
(1) ���ݷ����ɵã�100g����Һ�к��ȵ����ʵ���=![]() =
=![]() =0.05mol��������ԭ���غ㣬NaClO�����ʵ���Ϊ0.05mol����NaClO������Ϊ0.05mol��74.5= 3.725g��
=0.05mol��������ԭ���غ㣬NaClO�����ʵ���Ϊ0.05mol����NaClO������Ϊ0.05mol��74.5= 3.725g��
�ʴ�Ϊ3.725g��
(2) ��84����Һ����NaClO �����ʵ���Ũ��=![]() =
=![]() = 4.0mol/L�� n(NaClO)=1L��4.0 mol/L=4.0 mol�����ݷ�ӦCO2+NaClO+H2O�TNaHCO3+HClO������ҪCO2�����ʵ���Ϊn(NaClO)=4.0 mol������״����V(CO2)=4.0 mol��22.4 L/mol=89.6 L��
= 4.0mol/L�� n(NaClO)=1L��4.0 mol/L=4.0 mol�����ݷ�ӦCO2+NaClO+H2O�TNaHCO3+HClO������ҪCO2�����ʵ���Ϊn(NaClO)=4.0 mol������״����V(CO2)=4.0 mol��22.4 L/mol=89.6 L��
�ʴ�Ϊ��89.6��
��3��A������������ƽ����NaClO���壬�����ձ����ܽ�NaClO�����ò��������н������������������ƿ�ͽ�ͷ�ι������ݣ�ͼʾ��A. B. C. D����Ҫ�������貣�����ͽ�ͷ�ιܣ���A��ȷ��
B. ���ƹ�������Ҫ����ˮ�����Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�ã���B����
C. ����NaClO�����տ����е�H2O��CO2�����ʣ�������ƷNaClO���ܲ��ֱ��ʵ���NaClO���٣����Ƶ���Һ�����ʵ����ʵ�����С�����ƫ�ͣ���C��ȷ��
D. Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480 mL���ɣ�������ҪNaClO��������0.5 L��4.0 molL1��74.5 gmol1=149 g����D����
�ʴ�Ϊ��AC��
��4����ƽ���̵�����������������������������������������������30g�������������2.6g,���ձ�����������30.0g-2.6g=27.4g��
�ʴ�Ϊ��27.4g��
��5��������Һʱ��c=![]() ��������
��������
A��δ�ָ������¾ͽ���Һע������ƿ�����ж��ݣ���ʹVС��Ũ��ƫ��A����
B������Ͳ��ȡŨ����ʱ���Ӱ�Һ�棬��ʹnƫС��Ũ��ƫС����B��ȷ��
C������ƿ������ˮϴ��δ������滹Ҫ���ݣ�����Ӱ�죬��C����
D��δϴ���ձ��Ͳ���������ʹnƫС��Ũ��ƫС����D��ȷ��
E������ʱ����Һ�棬��ʹVƫ��Ũ��ƫС����E��ȷ��
�ʴ�ΪBDE��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ˮ���Ե����ʻ�������ˮ�γɽ��塣
(1)��������ˮ���γɵķ�ɢϵ��K2SO4��Һ��ͬ�߱���������_______��
a.���ȶ�����ж������Ա仯 b.���߾��ж�������� c.��ɢ�����ӿ�ͨ����ֽ
(2)��������0.5mol��L-1 K2SO4��Һ480mL��
��������Һʱ����������У�������ƽ��ҩ�ס��ձ�����������__________��__________���Լ��������ļ�Ƭ��ֽ��
�����Ƹ���Һ���ȡK2SO4���������Ϊ_____________
�����й�������ƿ��ʹ�÷����У���ȷ����________
A.����ƿ�ɳ��ڴ����Һ B.������ƿ��ֱ���ܽ����
C.��Һδ����ȴ��ע������ƿ�� D.������ƿ��ת����ҺҪ�ò���������
�����в�����ʹ������ҺŨ��ƫ�͵���___________
A.ϴ��������ƿƿ��������ˮ B.����ʱҩƷ������ŷ�
C.����ʱ���ӿ̶��� D.��Һ���ձ��Ͳ�����δϴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������dz��������ֽ��������ǵĵ��ʼ������������������д����ɼ���
��1������������ϡ���ᷢ����Ӧ��������![]() ��
��![]() ��д���÷�Ӧ�Ļ�ѧ����ʽ_______________��
��д���÷�Ӧ�Ļ�ѧ����ʽ_______________��
��2��ʵ��������![]() ��Һʱ�����û�и���������
��Һʱ�����û�и���������![]() �ᱻ����Ϊ________����д��ѧʽ�����ڸ���Һ�м���________�Լ�������_________����֤����Һ���ʡ�д������
�ᱻ����Ϊ________����д��ѧʽ�����ڸ���Һ�м���________�Լ�������_________����֤����Һ���ʡ�д������![]() �Ѿ������������ӷ���ʽ_________��
�Ѿ������������ӷ���ʽ_________��
��3����֪![]() ������ˮ����ͼ��ʾ������
������ˮ����ͼ��ʾ������![]() ��Һ����μ���
��Һ����μ���![]() ��Һʱ�����ɳ��������ʵ���y�����
��Һʱ�����ɳ��������ʵ���y�����![]() �����ʵ���x�Ĺ�ϵ��
�����ʵ���x�Ĺ�ϵ��
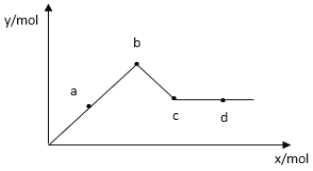
��ͼ��֪c��ij�����______���ѧʽ����a-b�����г������ʵ����ϴ����____���ѧʽ��,д���ӿ�ʼ�μ���![]() ��Һ��c����ܷ�Ӧ���ӷ���ʽ_______��
��Һ��c����ܷ�Ӧ���ӷ���ʽ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��M��R��Ϊ����Ԫ�أ���֪M��һ��ԭ��ʧȥ2�����ӣ�R��һ��ԭ�ӵõ�1�����Ӻ��γ�ϡ������Ԫ�صĵ��Ӳ�ṹ�����й���M��R�γɵĻ������������ȷ����(����)
A. M��R�����γ�MR2�����ӻ�����
B. ��MR2��M�����Ӱ뾶��R�����Ӱ뾶��
C. �γɵ��������ӷֱ���M����R2��
D. MR2�ĵ���ʽΪ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪KMnO4��ŨHCl�ڳ����·�Ӧ�ܲ���Cl2��������ͼ��ʾ��ʵ��װ�����Ʊ��������������������������������ķ�Ӧ��ÿ�����߿��ʾһ����Ԫװ�ã����д�����ǣ� ��

A.ֻ�Тٺ͢ڴ�B.ֻ�Тڴ�C.ֻ�Тں͢۴�D.ֻ�Тڢܴۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ������̼����ȼ�ϵ��ԭ��ʾ����ͼ�������йظõ�ص�˵����ȷ���ǣ� ��

A. ��ӦCH4��H2O![]() 3H2��CO,ÿ����1molCH4ת��12mol ����
3H2��CO,ÿ����1molCH4ת��12mol ����
B. �缫A��H2����ĵ缫��ӦΪ��H2��2OH����2e��=2H2O
C. ��ع���ʱ��CO32����缫B�ƶ�
D. �缫B�Ϸ����ĵ缫��ӦΪ��O2��2CO2��4e��=2CO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������в���ȷ����
A.ʵ����ȡ�ý����ƣ��������Ʒ������4��
B.���3.9gNa2O2�����к���m�������ӣ����ӵ�������ֵΪ20m
C.���н������仯��������ʱ���������ɫ����ɫ��Ӧ��Ӧ����ɫ�ܲ����۲�
D.K��KH������ˮ��Ӧ����H2��K2O2��KO2������ˮ��Ӧ����O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͷ���ؾ��������Ŀ������ã���ϳ�·����ͼ��ʾ��
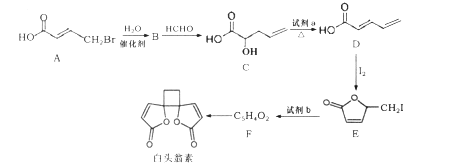
��֪��
��RCH2Br![]() RCH=CHR��
RCH=CHR��
��2RCH=CHR��![]()
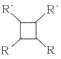
������R��R�������⡢�����
(1)��ͷ���صķ���ʽΪ____��
(2)�Լ�aΪ______��E��F�ķ�Ӧ����Ϊ________��
(3)F�Ľṹ��ʽΪ_________��
(4)C�к��еĹ���������Ϊ________��
(5) A��B��Ӧ�Ļ�ѧ����ʽΪ_________��
(6)F�����������ӳɵõ�G��G�ж���ͬ���칹�壬����������״��������____�֡�
(7)����ϩΪ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�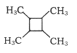 ��·��Ϊ____���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
��·��Ϊ____���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��M��X��N��Z��Yԭ��������������Ķ����ڵ���������Ԫ�أ�����X��Zͬ���壬Y��Zͬ���ڣ�M��X��Y�Ȳ�ͬ�壬Ҳ��ͬ���ڡ�Xԭ�������������Ǻ�����Ӳ�����������Y������ϼ�������ͻ��ϼ۵Ĵ����͵���6��N�Ƕ���������Ԫ����ԭ�Ӱ뾶���ķǽ���Ԫ�ء�
(1)��д������Ԫ�ص�Ԫ�����ƣ�X________��M________��
(2) Y�����ڱ��е�λ��______________��д��Z���⻯��ĵ���ʽ_____________
(3) N��������������������Һ��Ӧ�Ļ�ѧ����ʽ_________________��
(4)Y��Z������������Ӧˮ���������ǿ��˳��________��________(�û�ѧʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com