
| ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ����ϡ������������ˣ� | 20 | 20 | 20 | 20 |
| ��ַ�Ӧ��ʣ�������������ˣ� | 17.2 | 14.4 | 12.0 | m |
���� ��1��������Ӧ��ʵ�����ݣ����ݽ�����ԣ��Ͻ���ֻ�������������ᷴӦ�����������غ㶨�ɣ��ɵ�1��ʵ�����ݿ�֪��ÿ����20gϡ������ȫ��Ӧ��������20g-17.2g=2.8g���ݴ˷�������3�μ���20gϡ����ʱ����������14.4g-12.0g=2.4g��2.8g�����жϴ�ʱ�Ͻ��н���������ȫ��Ӧ�����ԣ���4�μ����ϡ�������������
��2��ʹ�ý�������ȫ��Ӧʱʣ�����ͭ��������������Ʒ�н�����������������������������Ʒ�������ȼ����ͭ��Ʒ����������������
��3��ʹ�õ�1��ʵ�����ݣ����������������������ݷ�Ӧ�Ļ�ѧ����ʽ������μӷ�Ӧ������������������������������ϡ����������ȼ�������ϡ���������������������
��� �⣺��1�����������غ㶨�ɣ��ɵ�1��ʵ�����ݿ�֪��ÿ����20gϡ������ȫ��Ӧ������20g-17.4g=2.6g���ݴ˷�������4�μ���20gϡ����ʱС��������12.2g-12.0g=0.2g��2.6g�����жϴ�ʱ�Ͻ��н���������ȫ��Ӧ�����ԣ���5�μ����ϡ��������Ľ��������ٷų���������˳�ַ�Ӧ��ʣ������������Ϊ12g��
�ʴ�Ϊ��12.0����12����
��2����ͭ��Ʒ��������������=$\frac{20g-12g}{20g}$��100%=40%��
�ʴ�Ϊ��40%��
��3��������ϡ�������������������Ϊx��������ã�
H2SO4 +Fe�TFeSO4+H2����1�֣�
98 56
20g��x 20g-17.2g=2.8g
$\frac{98}{20g��x}$=$\frac{56}{2.8g}$
x=24.5%
������ϡ���������ʵ���������Ϊ24.5%��
���� ��������ϡ�����������������ʱ��ע��ѡȡϡ������ȫ��Ӧʱ��ʵ�����ݼ�ǰ����ʵ������ݽ��м��㣮


 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
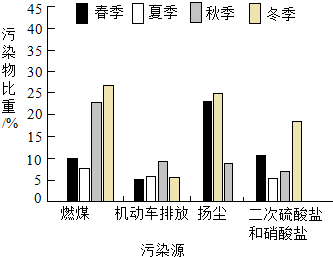 ��ͼΪ������PM2.5��Ⱦ����Դ�ļ��ڱ仯���������м��ڱ仯��������Ե���ȾԴ���ԣ�������
��ͼΪ������PM2.5��Ⱦ����Դ�ļ��ڱ仯���������м��ڱ仯��������Ե���ȾԴ���ԣ�������| A�� | ȼú | B�� | �������ŷ� | ||
| C�� | �ﳾ | D�� | ���������κ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cu | B�� | Fe | C�� | Cu��Zn | D�� | Cu��Fe |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���ʣ�������Ϊ���ʣ� | �����Լ� | ���뷽�� |
| A | �Ȼ��ƹ��壨̼���ƣ� | ϡ���� | �����ᾧ |
| B | ����������Һ������������Һ�� | ������̼���� | ���� |
| C | �Ȼ�ͭ��Һ���Ȼ�п�� | ����ͭ | ���� |
| D | �Ȼ��ƣ��Ȼ�þ�� | ��������������Һ | ���ˡ������ᾧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ����/g | |
| ���� | W |
| ����+FeSO4•xH2O | W1 |
| ����+FeSO4 | W2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
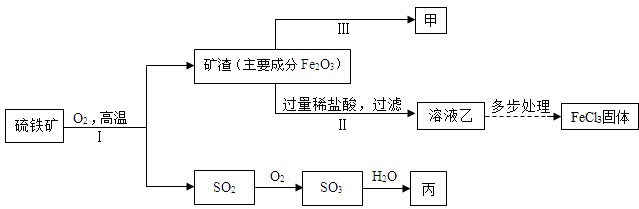
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com