
ʵ������ȡ���������װ������ͼ��ʾ����ش��������⣺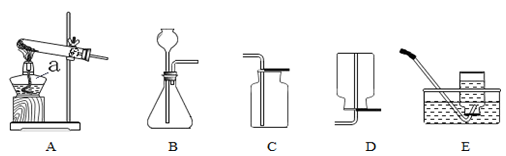
��1������a�������� ��
��2��ʵ������ȡ���巢��װ��ѡ��Ҫ���� �� ��
ʵ��������Aװ����ȡ�����Ļ�ѧ����ʽΪ ��ʵ��������Bװ���ƶ�����̼�Ļ�ѧ����ʽΪ ��
��3��ѡ�������ռ�����ʱ�����뿼�ǵ��������� ������ţ�����ɫ���ܶȢ��ܽ���
�ܿ�ȼ�ԣ��ռ�������̼��ѡ�õ�װ��Ϊ ������ĸ����ʵ���Ҽ��������̼��
������ ��������Ӧ�Ļ�ѧ����ʽ�� ��
��4������A��Eװ������ȡ�����������ܿ�����������ð��ʱ�����ռ���������ƿ��������
ð��ʱ��Ӧ���еIJ����� ��
��1���ƾ���
��2����Ӧ��״̬ ��Ӧ���� 2KMnO4 K2MnO4 + MnO2 + O2��
K2MnO4 + MnO2 + O2��
CaCO3 + 2HCl = CaCl2 + CO2�� + H2O
��3���ڢ� C ������������ͨ������ʯ��ˮ�������֤���Ƕ�����̼��
CO2 + Ca(OH)2 = CaCO3�� + H2O ��ȼ�ŵ�ľ���ŵ�����ƿ�ڣ�ľ��Ϩ��˵���ռ�����
��4����ˮ���ò���Ƭ��ס����ƿ���ó����������ϡ�
���������������2�����ݷ�Ӧ���״̬�ͷ�Ӧ����ѡ����װ�ã�װ��A�����ڹ̹̼����ͣ����Թܿ�����һС�������������ø��������ȡ��������ѧ����ʽΪ��2KMnO4��K2MnO4 + MnO2 + O2����Bװ�������ڹ�Һ�ڳ����·�Ӧ���������ô���ʯ��ϡ���ᷴӦ����ѧ����ʽΪ��CaCO3 + 2HCl = CaCl2 + CO2�� + H2O
��3���ռ�������ѡ���ǵ���������ܽ��Ժ�������ܶȣ�����ѡ��ڢۣ�������̼�ܶȱȿ�����������ˮ������ˮ��Ӧ������ֻ���������ſ�������ѡ�õ�װ��ΪC��ʵ���Ҽ��������̼��
�����ǣ�������������ͨ������ʯ��ˮ�������֤���Ƕ�����̼��������Ӧ�Ļ�ѧ����ʽ�ǣ�CO2 + Ca(OH)2 = CaCO3�� + H2O
��4�������ܿ�����������ð��ʱ�����ռ�����������ƿ��������ð��ʱ����ʾ���ռ�������ʱӦ��ˮ���ò���Ƭ��ס����ƿ���ó����������ϡ�
���㣺��������ķ���װ�ú��ռ�װ����ѡȡ������ʵ������ȡ�����ķ�Ӧԭ����ʵ�����ע�����ʵ������ȡ������̼�ķ�Ӧԭ����������̼�ļ��鷽����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(11��)��ͼ�г���ʵ������һЩʵ��װ�ã���ش��й����⣺ 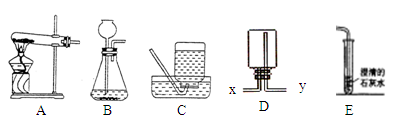
��1��װ��C�ɵ��ܡ�����ƿ�� �������������ƣ���ɡ�
��2��ʵ������H2O2��MnO2��ȡO2�Ļ�ѧ����ʽΪ ��ѡ�õķ���װ���� ������ţ���ͬ��������KClO3��MnO2��ȡO2����ѡ�õķ���װ���� ���ռ�װ�ÿ��� ��
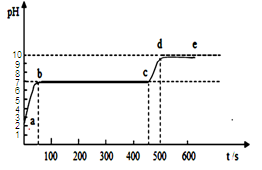
��3��ʵ������ȡ������̼�Ļ�ѧ����ʽΪ ��������̼����ͨ��E�У������������ǣ� ����ʯ��ʯ��ϡ������ȡ������̼ʱ��ѡ�õķ���װ���� ���������������ݳ���÷�Ӧ����Һ��pH��ͼ��a����ʾ���������Һ������̼������Һ����������ȷ�ⶨ��Һ��pH����ҺpH��ʱ��ı仯��ͼ������ʾ�������꣺pH�������꣺ʱ�䣩��
��д��bc�η�����Ӧ�Ļ�ѧ����ʽ�� �� cd ��������ԭ����: ��
��4��ʵ������ȡH2ʱ����ѡ��װ��D�ռ�H2����Ӧ�� �ڽ�������x��y����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���������ʵ������ȡ����ij���װ�ã��ش��й����⡣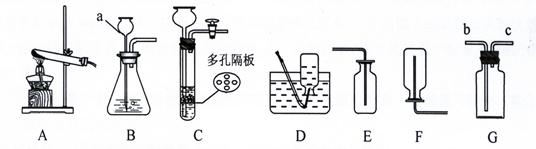
��1��д��ͼ�б�����ĸ���������ƣ�a ��
��2����A��E��Ͽ���ȡһ�����壬��Ӧ�Ļ�ѧ����ʽ
��
��3��ijͬѧ����Gװ�ô���Dװ�ã�����ˮ���ռ�һ�����������壬��ͬѧʹ��Gװ�õķ����� ��
��4��ʵ�������ÿ�״�����Һ�����������ȡ���壬�ɽ�Bװ�øĽ�ΪCװ�ã�����������ſ�״���壩�����ŵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijѧϰС��Χ�ơ�����ʵ������ȡ�����������֡��������������������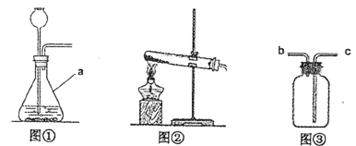
��1��ԭ��������ʵ�����ù���������Һ��ȡO2�Ļ�ѧ����ʽΪ ��
��2������װ�ã�ͼ��װ��������a�������� ��ʵ���ҳ�����ˮ�����ƹ������ʯ���ڼ��ȵ��������CH4��Ӧѡͼ ������ţ�����װ�á�
��3���ռ�װ�ã��ռ��ж�����SO2ʱ�������â��ռ�װ�ã�����Ӧ�� ������ĸ����ͨ�롣
��4��ʵ���������KMnO4��ȡO2�IJ������̿ɸ���Ϊ����װ������ ��װ��ҩƷ�������Թܡ��ռ������ֹͣ���ȡ���
��5��ʵ�鷴˼���ڼ���KClO3��O2�Ĺ����У����ֲ���O2�����ʺ���������鲻��KClO3���ʣ�Ҳ����װ�������Բ��ã�����Ϊ����ܵ�ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ʵ������ȡ�����װ��ͼ���밴Ҫ��ش��������⡣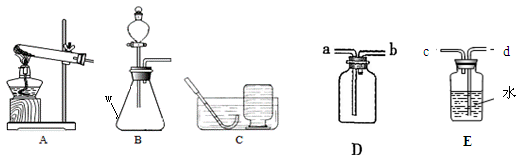
��1��д��ͼ�б�����������ƣ� ��
��2��ʵ�����Ʊ�CO2Ӧѡ�õķ���װ���� (����ĸ)���仯ѧ����ʽΪ ����װ�� ������ԡ������ԡ��������շ��������棻����Dװ���ռ�CO2��������Ӧ�� (��a��b )�˽���
��3��ʵ������H2O2��Һ��MnO2���������������MnO2�� ���ã��÷�
Ӧ�Ļ�ѧ����ʽΪ ������Eװ���ռ��������õ���O2���������ͲӦ�� ����c��d�������ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������װ�ã��ش����⣺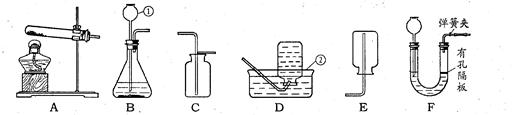
��1��д����Ţ٢ڵ��������ƣ���_____________����______________��
��2�������£���п��ϡ���ᷴӦ��ȡ������Ӧѡ�õķ���װ����________������ĸ��ţ���ͬ����ѡ��װ��___________���ռ����ϸ����H2��
��3��ʵ����ͨ����ϡ�����ʯ��ʯ��Ӧ��ȡCO2���÷�Ӧ�Ļ�ѧ����ʽΪ____________�����⣬CO2Ҳ������̼�����ƣ�NaHCO3��������ȷֽ⣨����Ϊ̼���ơ�������̼��ˮ������ȡ���÷�Ӧ�Ļ�ѧ����ʽΪ�� �����ô˷�����ȡCO2��Ӧѡ�õķ���װ����_________��ʵ���ҳ��� �����Լ����ƣ����������̼��������Ӧ�Ļ�ѧ����ʽΪ ���ж�CO2���ռ����ķ����� ��
��4���ø��������ȡO2��װ��A��ȱ�ٵ�һ����Ʒ��___________________��Ҫ�ռ�һƿ�ϴ��������������ѡ��װ�� ����װ����ţ���
��5����װ��F��ȡ���壬�ڷ�Ӧ�����У��õ��ɼм�ס�������ϵ���Ƥ�ܣ���һ�����Ӧ�ͻ�ֹͣ����ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʵ������ȡ���峣�õ�װ�ã���������ʻش��й�����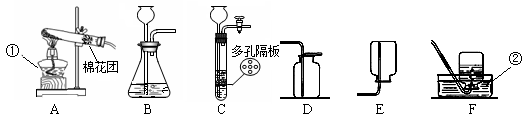
��1��д��������������ƣ��� ���� ��
��2�������Ѿ�ѧ��ʵ������ȡO2��H2��CO2��������Ļ�ѧ��Ӧԭ������д����ѧ��Ӧ����ʽ�� �� �� ��
ͨ���۲�Ƚϵó�����������ѧ��Ӧ�Ĺ�ͬ�� ������ţ���
A.��Ҫ���� B.��Ҫ���� C.û������μӷ�Ӧ D.���ɵ�����ֻ��һ��
��3��ʵ������ȡCO2�ķ���װ�ú��ռ�װ�÷ֱ��� ����װ�ô��ţ������������̼�ռ����ķ����� ��
��4������C��F��ϳ�װ������ȡ���壬Ӧ�������������е� �� ��
�ٷ�Ӧ���ǹ����Һ�� �ڷ�Ӧ����Ҫ����
���Ƶõ������ܶȱȿ���С ���Ƶõ����岻������ˮ
A���٢� B���٢ڢ� C���ڢ� D���٢ڢۢ�
��5��װ��C��B��ȣ��ŵ���
��6�����Ķ��±������Ϻش��������⣺
| ���� | ��ȡ�����ҩƷ | ��ȡ����ķ�Ӧ���� | ������������� |
| ���� | MnO2�����Ũ���� | ��Ҫ���� | ������ˮ���ܶȱȿ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ�־���ǿ�Ҵ̼�����ζ�����壬�ܶȱȿ���С����������ˮ����ˮ��Һ�Լ��ԣ�ʵ���ҿ��ü����Ȼ�����������ƹ�����������ȡ������
��1�������ͼ����ѡ��ʵ�����ô˷�����ȡ�����ķ���װ�� ��
��2������Cװ���ռ�������������Ҫ���� �����a����b�������뼯��ƿ��
��3���Ű���ʱ���ڲ�����Ӧע��ʲô���⣿
��4��ͼ�������ð�������һ��Ȥζʵ�飨�г��豸����ȥ������ƿ��װ�и���İ�������ͷ�ι���װ��ˮ���ȹرշ��ţ�����ͷ�ι��е�ˮ������ƿ�ڣ�Ȼ����ţ�����������ƿ�п��ܳ��ֵ�����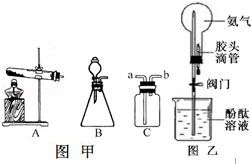
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ʵ���ҳ��ò���������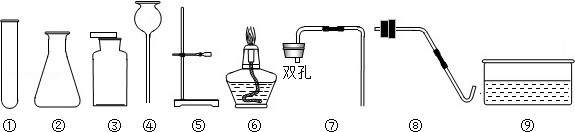
��1��ָ��ͼ�����������ƣ����� ����
��2��С��ͬѧѡ��������ȡ���ռ���������֪��ʹ���������٢ۢݢޣ���ô������Ҫʹ����ͼ�����е�
�� ������ţ����÷���װ�ü�������Եķ����ǣ��ѵ��ܵ�һ�˽���ˮ����ֽ����Թ���ڣ������ܿ����� ������˵����װ�ò�©����
��3��С��ͬѧ�����ռ�һƿCO2����֪�Ƿ��ռ����ˣ��������������� ����
��4��ij��ȤС��ѡ��������������п����ϡ������������п����Ӧ����ֻ��н϶�ĻҺ�ɫ�������ʣ��ӷ�Ӧ��Ļ�����з���õ��ûҺ�ɫ����IJ�����������
[�������]�Һ�ɫ�������ʵijɷ���ʲô�أ�
[�������]�ٿ��ܺ��е���̼���ڿ��ܺ��е���ͭ���ۿ��ܻ�����������
[���ʵ��]�����ʵ���û�ѧ����֤���ûҺ�ɫ�����������Ƿ��е���̼�͵���ͭ������±�����һ��Ҫ��������
| ���� | ʵ �� �� �� | �� �� �� �� �� |
| 1 | �� �� | �� |
| 2 | �� �� | �� �� |
| 3 | | |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com