
������ʵ������ȡ�����װ��ͼ���밴Ҫ��ش��������⡣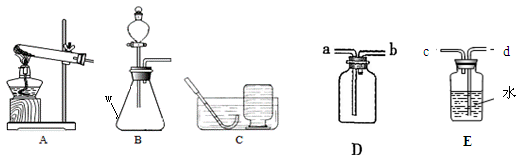
��1��д��ͼ�б�����������ƣ� ��
��2��ʵ�����Ʊ�CO2Ӧѡ�õķ���װ���� (����ĸ)���仯ѧ����ʽΪ ����װ�� ������ԡ������ԡ��������շ��������棻����Dװ���ռ�CO2��������Ӧ�� (��a��b )�˽���
��3��ʵ������H2O2��Һ��MnO2���������������MnO2�� ���ã��÷�
Ӧ�Ļ�ѧ����ʽΪ ������Eװ���ռ��������õ���O2���������ͲӦ�� ����c��d�������ӡ�
��1����ƿ
��4��B CaCO3+2HCl=CaCl2+CO2��+H2O ���� a
��3���� 2H2O2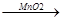 2H2O+O2�� d
2H2O+O2�� d
���������������ͼ֪���������w����������ƿ��ʵ�����Ʊ�CO2���õ�ҩƷ�ǿ�״�������ʯ��Һ��ϡ���ᣬ�Ҳ���Ҫ���ȣ���ѡB������װ�ã���ѧ����ʽ��CaCO3+2HCl=CaCl2+CO2��+H2O�����շ�����Ҳ�����ڿ�״������Һ�壬����Ҫ���ȵķ�Ӧ�����Կ��������շ��������棬������̼�ܶȱȿ�����������Dװ���ռ�CO2��������Ӧa���룬ʵ������H2O2��Һ��MnO2���������������MnO2������ã���ѧ����ʽ��2H2O2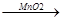 2H2O+O2������Eװ���ռ��������õ���O2���������ͲӦ��d�˽ӡ�
2H2O+O2������Eװ���ռ��������õ���O2���������ͲӦ��d�˽ӡ�
���㣺ʵ������ȡ������̼������������������װ�á��ռ�װ�á���ѧ����ʽ����д���顣


 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��4�֣���ͼ��ʵ����ȡ����ij���װ�á�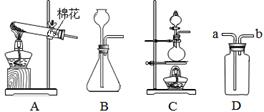
��д����Aװ����ȡ�����Ļ�ѧ����ʽ��
��2����ʵ���������Ũ��������������ڼ�����������Ӧ��ȡ������Cl2��������ȡ����Ӧѡ��ķ���װ���� ����װ�ñ�ţ���
��3������Dװ�ó�ȥCO2�л��е�ˮ��������װ����Ӧװ���Լ�Ϊ ��
����Ӧ�� ���a����b������ͨ�롣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͼ�ش����⣺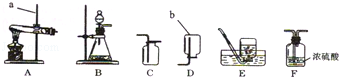
��1������a��b�����Ʒֱ����� ������ ��
��2��ʵ������ȡ������̼ʱ����ѡ�õķ���װ������ ������ĸ��ţ���ͬ������װ�õ��ŵ����� ����
��3��ʵ�����ù���������Һ�Ͷ���������ȡ�����Ļ�ѧ����ʽΪ�� �������������ڴ˷�Ӧ������ �����ã������ø÷�Ӧԭ����ȡ���ռ�һƿ�������������ѡװ�õ�����˳������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
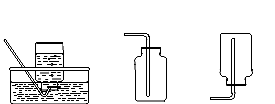
��ͼ��ʵ������ȡ���塢̽��������ɵij���װ�á�
��ش��������⣺
�������ٵ������� �������ڵ������� ��
����Aװ���м���˫��ˮ��Һ�Ͷ���������ȡ�������÷�Ӧ�Ļ�ѧ����ʽΪ ��
��Ϊ����֤ij�����������H2��CO��H2O�����壩��ɣ��������ʵ�飬ʵ����Bװ�õ������� ��֤����һ����̼���ڵ�ʵ�������� ���ᵼ�� ��
��������װ��������ȡ������̼�����ܿ��Ʒ�Ӧ�ķ�����ֹͣ���������շ�����ԭ������ͬ��װ���� ��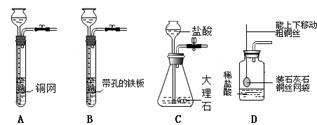
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij����������Ʒ�к�������̿��Ϊ�ⶨ����Ʒ�ж������̵�����������ij��ȤС�����������ʵ�鷽������һ��������Ʒ��ͨ����﴿����������ʹ����̿�ڼ��������·�Ӧ����CO2�����з����ⶨ��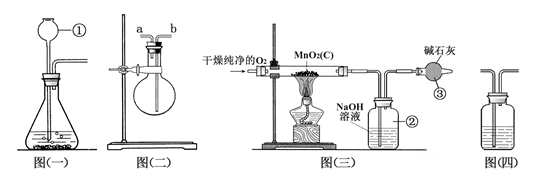
��1�������ٵ������� �������ʵ��ѡ��ͼ��һ��װ������ȡ������������Ӧ�Ļ�ѧ����ʽΪ �����ж������̵��� ���á�
��2����ͼ������װ�ÿ��ռ���������������ƿ����ˮ���ռ�����������Ӧ�� (��a����b������ͨ�롣
��3��ͼ���������ø��﴿����O2����Ʒ��Ӧ���ⶨ������������������װ�ã�װ�â���װ�м�ʯ�ң���������____________________________________________________��
��4��Ϊ��֤ͼ��������װ�â��ѽ�CO2������ȫ������װ�â����֮�����ͼ���ģ�װ�ý���֤������ͼ���ģ�װ���м�����Լ�Ϊ__________������ĸ����
A��NaOH��Һ������B������ʯ��ˮ��������C��Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ������ȡ���������װ������ͼ��ʾ����ش��������⣺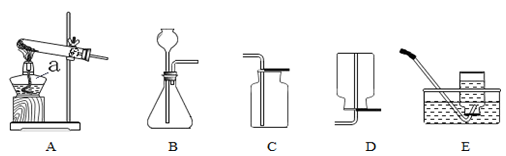
��1������a�������� ��
��2��ʵ������ȡ���巢��װ��ѡ��Ҫ���� �� ��
ʵ��������Aװ����ȡ�����Ļ�ѧ����ʽΪ ��ʵ��������Bװ���ƶ�����̼�Ļ�ѧ����ʽΪ ��
��3��ѡ�������ռ�����ʱ�����뿼�ǵ��������� ������ţ�����ɫ���ܶȢ��ܽ���
�ܿ�ȼ�ԣ��ռ�������̼��ѡ�õ�װ��Ϊ ������ĸ����ʵ���Ҽ��������̼��
������ ��������Ӧ�Ļ�ѧ����ʽ�� ��
��4������A��Eװ������ȡ�����������ܿ�����������ð��ʱ�����ռ���������ƿ��������
ð��ʱ��Ӧ���еIJ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
.ij��ѧ��ȤС��������ͼװ��̽����ȡ�����ԭ�������������ʡ����װ��ͼ���ش��������⣺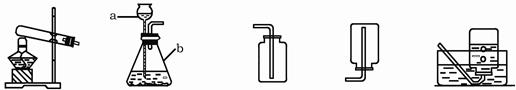
A B C D E
��1��д��ͼ�б�����������ƣ�a ��b ��
��2����˫��ˮ�Ͷ���������ȡ����ʱ����ѡ�õķ���װ���� (����ͼ��ĸ)����ѡ��Cװ���ռ���������ԭ���� ��
��3��ʵ���ҳ����Ȼ�粒������ʯ�ҹ��干������ȡ����(NH3)��Ӧѡ��ķ���װ��
�� (����ͼ��ĸ)��
��4����С�����������ͼ��ʾ��ʵ��װ�ã��ȿ�������ȡ���壬�ֿ�����̽���������ʡ�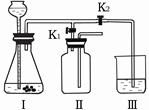
�ٵ���K1���ر�K2ʱ������I����װ�ã��ɽ��е�ʵ���� (����ĸ)��
a������ʯ��ϡ���ᷴӦ��ȡ������̼
b��п��ϡ���ᷴӦ��ȡ����
�ڵ���K2���ر�K1ʱ���������ù�����װ���Ƶö�����̼����֤�����ʡ�ʵ������ȡ������̼�Ļ�ѧ����ʽ�� ����Ҫ֤��������̼����ˮ������Ӧ��Ӧ���ձ���ˮ�м��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������ʵ�鳣��װ�ã��ش��й����⡣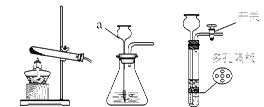
A B C D E F
��1��д������a�����ƣ� ��
��2����������غͶ���������ȡ�������÷�Ӧ�Ļ�ѧ����ʽ�� �����������ڴ˷�Ӧ�е������� ����װ��A��D���ӽ��д�ʵ�飬ʵ�������ֹͣ����ǰҪ�Ƚ������Ƴ�ˮ�棬Ŀ���� ��
��3�����ÿ�״ʯ��ʯ��ϡ������ȡ������̼��ѡ�õ�װ������� ������ĸ��ţ����ڼ��Լ�֮ǰ�IJ����� �����ռ����Ķ�����̼����ͨ��ʢ������ˮ���Թܣ�һ��ʱ���ø��Թ�����Һ��pH ���������������������7���Ը�����ĺ�������Ϊ ���û�ѧ����ʽ��ʾ����
��4��C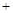 O2
O2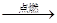 CO2����������Ϥ�ķ�Ӧ��Ϊʲô��ʵ�������Dz�����̼��ȼ������ȡCO2������Ҫԭ���� ��д�� 1������
CO2����������Ϥ�ķ�Ӧ��Ϊʲô��ʵ�������Dz�����̼��ȼ������ȡCO2������Ҫԭ���� ��д�� 1������
��5��ijͬѧ��ʯ��ʯ��ϡ������ɡ���ȡ������CO2����ʵ�飬������73��10%�����ᡣ����ʵ������в���CO2�����ʵ����Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪���ᣨH2C2O4��������Ũ���ṩ�ȷ�����ѧ��Ӧ��H2C2O4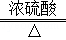 CO2��+CO��+H2O��ij��ѧ��ȤС��ͬѧ�����ͼ��ʾʵ��װ�ã�������ȡ���������CO��������ԭ���������������ĿҪ��ش��������⣺
CO2��+CO��+H2O��ij��ѧ��ȤС��ͬѧ�����ͼ��ʾʵ��װ�ã�������ȡ���������CO��������ԭ���������������ĿҪ��ش��������⣺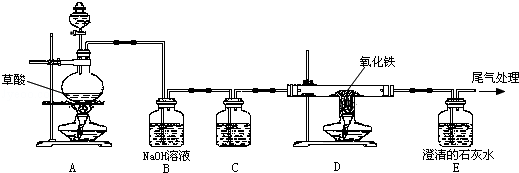
��1��ʵ�鿪ʼʱ��Ӧ���ȵ�ȼ�� ���ľƾ��ƣ���װ����ţ���
��2��C����ʢ��Һ������ ����
��3��Dװ�ò������й۲���������� ����
��4����Eװ�ó�����β��Ҫ���д�������δ����� ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com