
����Ŀ��(1)��֪ij���ӵĽṹʾ��ͼΪ��
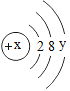
�Իش𣺢ٵ�x��y��10ʱ��������Ϊ________(����ԭ����������������������������)��
�ڵ�y��8ʱ�����ӿ���Ϊ��______(��һ�����ӷ���)��
(2)��Ca��Na��C��H��O��N��Fe����Ԫ����ѡ���ʵ���Ԫ�أ���Ҫ����ա�
���ʵ������ֺͷ�����գ�
��3����������______���ڰ����е�Ԫ�صĻ��ϼ�_____��
д����������Ҫ������ʵĻ�ѧʽ��
�����������ЧӦ��������______����һ�����������ࡢ����ȼ��_____��
�ۺ決����õķ��ͷ��к��е�����_____������Ϊ���������õļ�_____��
���𰸡�ԭ�� K+�� 3Fe2+ ![]() CO2 H2 NaHCO3 Ca(OH)2
CO2 H2 NaHCO3 Ca(OH)2
��������
�����������ͺ���������Ƿ�����ж����ӵ����ͣ����������к��������������ں�����������ó����ӵĺ����������������ж��������ࣻ���ӵı�ʾ�������ڱ�ʾ�����ӵ�Ԫ�ط������Ͻǣ���������������������������������ǰ���������ں�1�����ʱ��1Ҫʡ�ԣ�ȷ������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص����Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�
��1����x-y=10��˵��x=10+y�����Ϻ˵����=������=�����������������ԭ�ӡ��ʴ�Ϊ��ԭ�ӣ�
��ԭ���У��˵����=������=�����������ԭ�ӵ�ʧ���ӿ����γ�8�����ȶ��ṹ��ϡ��������8�����ȶ��ṹ�����Ͻṹ��ԭ��ΪAr��������Ϊ��K+��Ca2+��������Ϊ��S2-��Cl-�ȡ��ʴ�Ϊ��K+�ȡ�
��2���������������ӣ��������Ӵ�������λ������ɣ�������ǰ��д���ֱ�ʾ���ӵĸ�������3Fe2+��������һ�ֻ����Ԫ�ػ��ϼ۴�����ӦΪ0���赪Ԫ�صĻ��ϼ���x��HԪ�صĻ��ϼ�ͨ��Ϊ+1����x+��+1����3=0�����x=-3���������е�Ԫ�ػ��ϼ�Ϊ-3���ʴ�Ϊ��3Fe2+��![]() ��
��
���������ЧӦ�������Ƕ�����̼����ѧʽΪCO2���ʴ�Ϊ��CO2��
��һ�����������ࡢ����ȼ��������������ȼ��ֻ����ˮ����������Ⱦ���ʴ�Ϊ��H2��
�ۺ決����õķ��ͷ��к��е�����̼�����ơ��ʴ�Ϊ��NaHCO3��
����Ϊ�������ϣ��õļ����������ơ��ʴ�Ϊ��Ca(OH)2��
�ʴ�Ϊ����1����ԭ�Ӣ�K+�ȣ�2����3Fe2+��![]() ��CO2��H2��NaHCO3��Ca(OH)2��
��CO2��H2��NaHCO3��Ca(OH)2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��С������ͼ����ʵ�飬��ش��������⣺
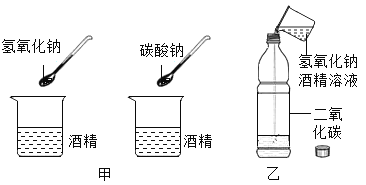
С��ͬѧ�������°���ͼ����ʾ���ֱ���ʢ����������ƾ����ձ��м�����������������ƣ�̼���ƽ���ʵ�飬�۲쵽�����������������ھƾ�����ȫ�ܽ⣬̼�����ھƾ����ܽ�ĺ��٣������õ��������ƾƾ���Һ����ʢ�ж�����̼������ƿ�У���ͼ����ʾ�����Ǻ�ƿ�ǣ����۲쵽ƿ����Һ����ǣ�����ƿ____________��ƿ����Һ����ǵ�ԭ����________________����Ӧ�Ļ�ѧ����ʽΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪʵ�����г����������Ʊ����������ռ�������ʵ��IJ����������Ը�����ĿҪ�ش��������⣺
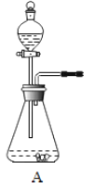
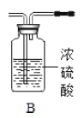
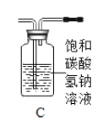
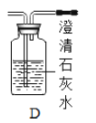
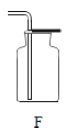
��1������ʯ��ʯ��ϡ����Ϊԭ�ϣ���ʵ�������Ʊ����ռ�һƿ���﴿���Ķ�����̼���塣
����ѡ����������˳��Ϊ__________������������д���������ĸ����
����д��װ��A����������Ӧ�Ļ�ѧ����ʽ_________��
�����ʾ�����;�����й��ڶ�����̼����;��Ҫ�������������ʵ���_______����д���A��B��C��D֮һ����
A ������̼���������
B ���������̼�������˹�����
C ������̼�������������
D ������̼����������̼������
��2���ڶ����ͬѧ�Թ���������ҺΪԭ�ϣ�![]() Ϊ�������Ʊ�����������ij�����ϵ����Ԫ�ؽ��з���̽����������ʾ������ֻ��
Ϊ�������Ʊ�����������ij�����ϵ����Ԫ�ؽ��з���̽����������ʾ������ֻ��![]() ����Ԫ�أ�����ѡ��������
����Ԫ�أ�����ѡ��������![]() ��ʯ�Ҹ��������˳�����ӣ���
��ʯ�Ҹ��������˳�����ӣ���![]() ΪŨ����ϴ��ƿ����ʵ��ǰ����װ�������ԣ�ʹ������������м�ڴ������г��ȼ�գ��۲������ռ��й�ʵ�����ݣ����跢���Ļ�ѧ��Ӧ����ַ�Ӧ�����Իش��������⣺
ΪŨ����ϴ��ƿ����ʵ��ǰ����װ�������ԣ�ʹ������������м�ڴ������г��ȼ�գ��۲������ռ��й�ʵ�����ݣ����跢���Ļ�ѧ��Ӧ����ַ�Ӧ�����Իش��������⣺
������A����������Ӧ�Ļ�ѧ����ʽΪ��____________��
�ڵ�����D�г���_________������ʱ�����֤��������Ʒ�к���̼Ԫ�ء�
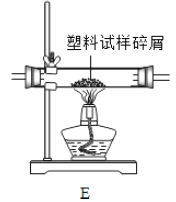
������E�IJ������з��������������м����Ϊmg�������������ȼ�պ������![]() ��������ag������
��������ag������![]() ��������bg������D��������cg����mg�����������к���Ԫ�ص�����Ϊ________g��̼Ԫ�ص�����Ϊ_______g����������Ϊ������ʽ����
��������bg������D��������cg����mg�����������к���Ԫ�ص�����Ϊ________g��̼Ԫ�ص�����Ϊ_______g����������Ϊ������ʽ����
����װ����û����������![]() ������������ĸ�����������̼Ԫ�ص�������ʵ��ֵ�ȽϽ�__________������ƫС����ƫ����������һ����֮һ����
������������ĸ�����������̼Ԫ�ص�������ʵ��ֵ�ȽϽ�__________������ƫС����ƫ����������һ����֮һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼΪ�ס��ҡ����������ʵ��ܽ�����ߡ�����˵����ȷ����

A����t1��ʱ���ܽ������������
B������������Һ�������϶����ľ���ͨ���ɲ��ý��½ᾧ��
C�����������ס���������Һ��t3�潵�µ�t1�棬�������壨�ᾧˮ��������������
D��t2��ʱ�����������ı�����Һ������t3��, ����Һ����������������������Һ����������������С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼��þˮ�������Ʊ�þ��Ʒ���м��壮
����ȡMgCO33H2O����ҵ�ϴ�������±ˮ(��Ҫ�ɷ�ΪMgCl2)��ȡMgCO33H2O�ķ�����ͼ1��
(1)�������̵Ļ�ѧ����ʽΪ��MgCl2+CO2+2NaOH+2H2O�TMgCO33H2O��+2____��
(2)�������̵�pH��ʱ��ı仯��ͼ2��ʾ���������̵IJ���Ϊ___(����ĸ)��
a ��±ˮ�еμ�NaOH��Һ��ͬʱͨ��CO2
b ��NaOH��Һ�еμ�±ˮ��ͬʱͨ��CO2
c ��±ˮ��ͨ��CO2�����ͣ�Ȼ��μ�NaOH��Һ��ͬʱ����ͨ��CO2
d ��NaOH��Һ��ͨ��CO2�����ͣ�Ȼ��μ�±ˮ��ͬʱ����ͨ��CO2
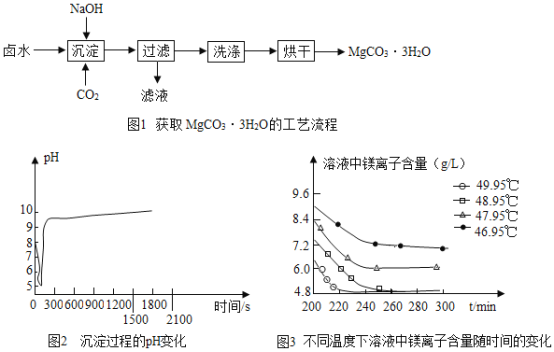
(3)�������̵���Һ��þ���Ӻ�����ʱ��ı仯��ͼ3��ʾ����ͬ�¶������õ������������±���ʾ�����������ѡ����¶�Ϊ____��������_______��
�¶�(��) | ���� |
46.95 | MgCO33H2O |
47.95 | MgCO33H2O |
48.95 | MgCO33H2O |
49.95 | Mg5(OH)2(CO3)44H2O |
(4)���ⶨMgCO33H2O�Ĵ��ȣ�������ͼ����ʵ�飬��ȷ��MgCO33H2O�Ĵ��ȣ�
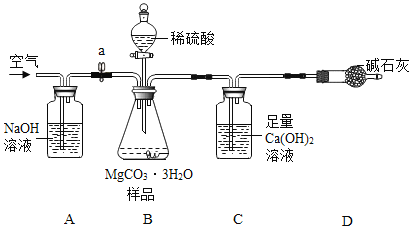
����ʵ����̻ش��������⣺
(5)ʵ����������������ͨ������������ó��˿ɽ���B��C�еķ�Ӧ���⣬����____��
(6)C�л�ѧ����ʽΪ___��A��NaOH����Ϊ___��D�м�ʯ������Ϊ____��
(7)���и����ʩ�У�������߲ⶨȷ�ȵ���___(����)��
a �ڼ�������֮ǰ��Ӧ�ž�װ���ڵ�CO2���� b Ϊ������ʵ��ʱ�䣬���ٵμ�����
c ��A��B֮������ʢ��Ũ�����ϴ��װ�� d ��Cװ����ർ��ĩ�������������
(8)ʵ����ȷ��ȡ15.0g��Ʒ���ݣ��������βⶨ���������CaCO3������ƽ������Ϊ10.0g���������Ʒ��MgCO33H2O�Ĵ���___(д���������)��
(9)С����ΪӦ������ʯ��ˮ����Ba(OH)2��Һ�������ɳ���Ba(OH)2�ܽ�ȴ�Ũ�ȴ�ʹCO2�����յĸ���ȫ�⣬����____��
(10)����ȡMgCO33H2O����Ʒ�к�������Mg5(OH)2(CO3)44H2O�����Ʒ��þԪ�ص���������__(����ƫ����������������ƫС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨijAgNO3��Һ������������������������������Ϊ7.3%��ϡ������еζ�����ȡ����Һ50.0g���ζ�������ͼ��ʾ��(������������1λС��)
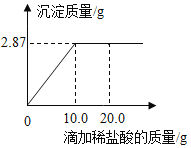
(1)��AgNO3��HClǡ����ȫ��Ӧʱ������7.3%��ϡ���������Ϊ��____��
(2)��ϡ����������μ���20.0gʱ����Һ�е�����Ϊ_____(д����ѧʽ)
(3)����AgNO3��Һ��������������_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧС��ͬѧ�ڼ��ȶ���������Ʒʱ�����������ݲ�������ͼһ��ʾ�����������Ƕ�����쳣���������̽����
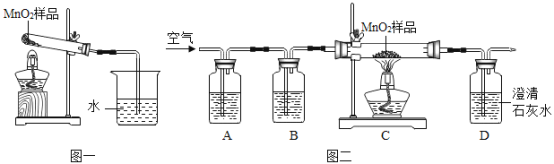
��1��������ɷֵ�̽�������ȶ���������Ʒ��������������ͨ������ʯ��ˮ������ʯ��ˮ��������ӵ㲿�ַ�Ӧ�Ļ�ѧ����ʽ��_____________ ���ɴ˿�֪�������������Ƕ�����̼��
��2����������Դ��̽����
������1����ͬѧ��Ϊ���������������Թ��еĿ������������ʵ��֤���ü��費����
ʵ�鲽�� | ʵ������ | ���� |
�Թ��в��Ŷ������̣�����Թܣ�����ͼһװ�ý��м��ȣ�������ͨ�����ʯ��ˮ�� | ___________ | ���費���� |
������2����ͬѧ��Ϊ������������Ʒ�п��ܻ���̿�ۣ�̿�۷�����Ӧ�����˸����塣
��ʵ����ƣ����������ͼ����ʾ��ʵ������о�������Aװ����ʢ������������Һ�����ڳ�ȥ�����еĶ�����̼���壬Bװ�õ������Ǽ���A���Ƿ���ȫ����������̼���壬��B��Ӧʢ�ŵ��Լ���_____________��ʵ���й۲쵽B�������Ա仯��D������ʯ��ˮ����ǡ�
�����õ�����������������������ʵ���е��κη�Ӧ�����ظ�����ʵ�飬����B�������Ա仯��D�������ʯ��ˮ������ǡ�
�����ۣ�ͨ����ͬѧ��ʵ��֤�������������л���̿�ۣ�̿��������е�____________��Ӧ�����˶�����̼���塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��ͬѧ�����˼������ʯ��ˮ������������Һ��ʵ��̽��������һͬ����̽����

��������⣩��μ�����������ɫ��Һ��
��ʵ�鷽���������ȼ�λͬѧ��������ͼ��ʾ��ʵ�飬����ش��������⣺
��1�����в��ܴﵽʵ��Ŀ�ĵ���________������ĸ��
��2��д��C��ʵ���з�����Ӧ��ʵ������_________________��
��3��д��D��ʵ��������������Ļ�ѧ����ʽ______________��
������̽����ʵ�����������ͬѧ��A��B��C��D�����Թ��е�����ȫ������ͬһ���ɾ����ձ��У���ַ�Ӧ�õ���ɫ����������Һ���Ը���Һ�ijɷ��ֽ�����̽����
��������⣩����Һ�г�ˮ����̪�������Щ���ʣ�
���������ϣ��Ȼ�����Һ�����ԣ�
����������裩��NaCl��CaCl2 ��NaCl��CaCl2��NaOH ��NaCl��CaCl2��HCl
����˼����չ��
�����������������ֻ��һ��������������________������ţ���������________��
��Ϊ����֤�ձ�����Һ�п����е������Ƿ���ڣ�ʹ��������Щ���ʿ��ԴﵽĿ����________������ĸ����
A ������ B ͭ C ��������Һ D ��������������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ������֪��������̼��ˮ��Ӧ����̼�ᣬ��ô����������ˮ�Ƿ�Ҳ�ܷ�Ӧ����һ�����أ�ijʵ��С��Դ˽���̽������Ƶ�̽���������¡�����ش����е��й����⣺
��1���������裺_______________________��
��2����Ʒ���������֤ˮ�ܷ�ʹ��ɫʯ����ֽ��ɫ������֤�������������ܷ�ʹ�������ɫʯ����ֽ��ɫ�������֤�������������ܷ�ʹʪ�����ɫʯ����ֽ��죬ʵ��װ�ú�ҩƷ��ͼ��
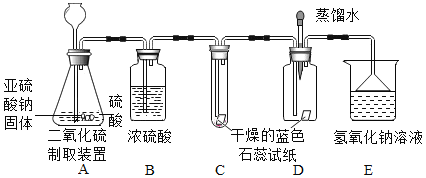
��3���������ϣ�����Ϊ��ʵ��С����Ҫ���յ�����������Ӧ����������ţ�_________
�ٶ�������������ˮ ������ʹʪ�����ɫʯ����ֽ��� ��SO2����Ũ���ᷴӦ �ܶ��������ж����������Һ��Ӧ�����κ�ˮ
��4��ʵ�飺
��ʵ�������װ��C��ʯ����ֽ����ɫʼ��û�б仯����˵��__________________��
��װ��D�н�ͷ�ι��е�����ˮ�ڶ���������������֮ǰ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯�����ж�����������ͨ��ʱ����ʪ�����ɫʯ����ֽ��졣������˵��_________________���˹����з�Ӧ�Ļ�ѧ����ʽΪ______________________��
��װ��E��������_____________________��
��5�����ۣ�ԭ����_______________ (����������������)��
������Ƥ�����ҹ���ͳ�ĵ���Ʒ����������ϲ���������� NaCl��CaO ������ Na2CO3 ��ϵõ��ķ�ĩ״����� A ����Ƥ�����䷽֮һ��
��1��ij��ѧ��ȤС����г���ȡ�����Ļ���� A ���ձ��У�������������������ˮ���ӱ߽��裬���ã����˵���Һ����Һ�к��� Cl- ��OH- �������е���������__________ ��
��2���ڻ����A�м���������ZnSO4�ɸ���Ƥ��Ʒ�ʣ�����ȤС����ij��Ʒ��̽����������Ƿ������������� ZnSO4��
���ʵ�鷽��������������� ��ѡ��Ʒ���Լ���ϡ���ᡢpH ��ֽ��BaCl2 ��Һ������ˮ��AgNO3 ��Һ
����1��ȡ������Ʒ���ձ��У�������������������ˮ����ֽ��裬���ã����ˡ� | |
����2��ȡ��������1�γɵ���Һ���Թ��У������м�������ϡ���ᣬ�� | ________________ |
����3��______________ | __________��˵����Ʒ����ZnSO4�� __________��˵����Ʒ��û��ZnSO4�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com