
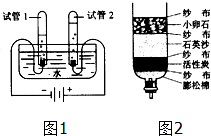
 ˮ������֮Դ������Ӧ���˽�ˮ������ˮ��Դ��
ˮ������֮Դ������Ӧ���˽�ˮ������ˮ��Դ������ ��1��Ӧ�ù������ʵ���֮����ڼ���Ĺ۵������ʹ�����
��2�����ݵ��ˮʵ�������ͽ��۷����ش�
��3��������ɷ������
��4������Ӳˮ�����ķ��������ش�
��5�������ھ�ˮ����С��ʯ��ʯӢɳ�������ޡ�����̿���ص㼰���÷����ش�
��� �⣺��1��ˮ�;ƾ������ɷ��ӹ��ɵ����ʣ����ڹ������ǵķ���֮�䶼����һ���ļ�����������ʱ���ַ����ռ���˿ռ䣬�����������С�ڶ��ߵ����֮�ͣ�˵���˷��Ӽ���ڼ����
��2���ɵ��ˮʵ���װ�ÿ�֪�������Թ�2�е�������ٿ�֪��������������������������������Ϊ1��2��ͨ��ѧ��Ӧǰ��Ԫ�ص�����䣬����������������Ԫ����ɣ���������Ԫ����ɣ�����֪��Ӧ��ˮ���⡢������Ԫ����ɵģ�
��3����Ȫˮ������ˮ����ˮ�ж��������ʣ����ڻ�������ˮ����һ��������ɵ����ڴ����
��4��С����ϴ�·�ʱ����ϴ�����·��ر����ױ�Ӳ�����ƣ������˷ѷ�����ϴ���ɾ���˵���˴�ˮΪӲˮ��Ӧ����������������������ͨ����з�������������⣮
��5���ڼ���ˮ���У�С��ʯ��ʯӢɳ���������ܳ�ȥ�����Ե����ʣ��������ǹ��ˣ�����̿�������ԣ�������������
�𰸣���1�����Ӽ���ڼ����
��2��������1��2��ˮ������Ԫ�ء���Ԫ����ɣ�
��3������ˮ��
��4�����
��5�����ˣ�������
���� ����Ƚ�ȫ��ؿ�����ˮ����ɡ�������Ӳˮ�����������Ӽ��м����֪ʶ���漰��֪ʶ��϶࣬���ѶȲ������ڻ�����֪ʶ��Ӧ��ǿˮ��֪ʶ�Ĺ��ɺ�ѧϰ��


 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ���ۻ� | B�� | ��ʪ���·���ɹ���� | ||
| C�� | �������� | D�� | ˮ���ˮ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �� ʵ | �� �� |
| A��H2O2��ɱ����������H2O���� | �������ʵķ��ӹ��ɲ�ͬ |
| B���¶ȼ��е�ˮ���������������� | ԭ�ӵĴ�С�����ı� |
| C�������ױ�ѹ�� | ������Ӽ�ļ���ܴ� |
| D���������� | �����ڲ����˶� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2����� | |
| I | 50.0g | 1% | 0.1g | 9mL |
| �� | 50.0g | 2% | 0.1g | 16mL |
| �� | 50.0g | 4% | 0.1g | 31mL |
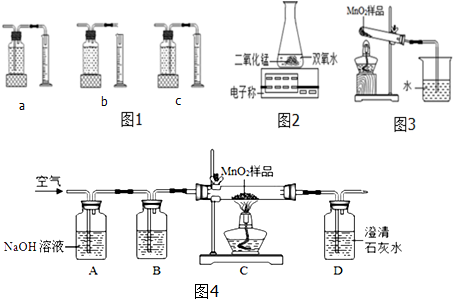
| ʵ�鲽�輰���� | ���� |
| ��ͼ3װ�ã����ȿյ��Թܣ���һ�˵ij���ʯ��ˮû�б���� | ���費������������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | a��ȼ�ŵ�ľ��Ϩ��b��ȼ�ŵ�ľ���Ļ������ | |
| B�� | a��ȼ�ŵ�ľ���Ļ��������b��ȼ�ŵ�ľ��Ϩ�� | |
| C�� | a��b��ȼ�ŵ�ľ���Ļ��涼���� | |
| D�� | a��b��ȼ�ŵ�ľ����Ϩ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com