
����Ŀ��С�մ����Ҫ�ɷ���̼�����ƣ��������������������Ȼ��ƣ���ѧ��ȤС���ͬѧҪͨ��ʵ�����ⶨijƷ��С�մ���Ʒ��̼�����Ƶ�����������
(1)ȷ��ȡ9.0gС�մ���Ʒ�����ձ��У���μ�����������Ϊ5%��ϡ������ǡ�ò��ٲ�������Ϊֹ��������ϡ����73.0g���ձ���û�в��������������Ʒ��̼�����Ƶ�����������(д���������)____
(2)�������һ��������ʵ��ԭ���Ͳ�������������ͬ��ʵ�飬�ⶨС�մ���Ʒ��̼�����Ƶ���������_______��
���𰸡�93.3%ȷ��ȡngС�մ���Ʒ�����Թ��У����������ٲ�������Ϊֹ��ȷ����ʣ����壨Na2CO3������Ϊmg�����÷���ʽ�ɼ�����Ʒ��̼�����Ƶ������������������������������ɣ�
��������
��̼�����Ƶ�����Ϊx
NaHCO3 �� HCl=== NaCl��H2O��CO2��
84���� 36.5
x������ 73.0g��5%
![]() =
=![]()
x��8.4 g
��Ʒ��̼���Ƶ���������Ϊ![]() ��100%��93.3%
��100%��93.3%
����Ʒ��̼�����Ƶ���������Ϊ93.3%��
��2��ȷ��ȡngС�մ���Ʒ�����Թ��У����������ٲ�������Ϊֹ��ȷ����ʣ����壨Na2CO3������Ϊmg�����÷���ʽ�ɼ�����Ʒ��̼�����Ƶ������������������������������ɣ�
����Ʒ��̼�����Ƶ�����Ϊx
2NaHCO3![]() Na2CO3+H2O+CO2��
Na2CO3+H2O+CO2��
168 18+44
x n-m
![]() =
=![]()
x=![]() ����n-m��
����n-m��
С�մ����������Ϊ![]() ����n-m����n��100%
����n-m����n��100%


 һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˲ⶨij����ͭ��Һ������������С��ͬѧȡ50��0g��Ʒ�����ձ��У���μ�������������Һ����������������Һ���������ɳ��������Ĺ�ϵ����ͼ��ʾ������㣺
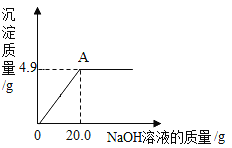
(1)NaOH�Ļ�ѧʽ��Ϊ_________��
(2)����ͭ�պ���ȫ��Ӧʱ��������Һ������Ϊ_____g(����һλС��)��
(3)����ͭ��Һ�����ʵ�����������________��(д���������)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ľ��(��Ҫ�ɷ�ΪK2CO3�����ʲ�����ˮ)��Ʒ�����ܻ�������K2SO4��KCl��ij��ѧ��ȤС�����������ʵ�飺
(1)��Ʒ��Ԥ������

�ٲ���a������Ϊ__________________����Ŀ����____________________
����ҺA����Ҫ�������ӣ���CO32-�⣬���ܻ�����_______________(�����ӷ���)
(2)��Ʒ�ɷֵ�ȷ�������ʵ�鷽�������������������ѡ�Լ���ϡ���ᡢϡ���ᡢϡ���ᡢ�Ȼ�����Һ�����ᱵ��Һ����������Һ
ʵ����� | Ԥ������ͱ�Ҫ���� |
����1��ȡ��ҺA�������Թ��У��������� _______________����������ã����ˡ� |
|
����2��ȡ��������1���õ���Һ���Թ��У�____________ | ______��˵����Ʒ��δ����KCl |
����3��ȡ��������1���õ��������Թ��У�__________________ | ______��˵����Ʒ��������K2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������װ�ö���Ļ�ѧ���ʽ���̽����
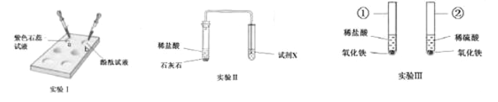
��1�����Ϊ�ٵ�����������_____��
��2��ʵ��I�У��ڵ�ΰ��a��bѨ�е�������Һ����Һ������_____���a����b������ʹ�õ�ΰ���ŵ�֮һ��_____��
��3��ʵ����У�Ϊ����֤ʯ��ʯ�к���̼������ӣ��Լ�X��������_____��
��4��ʵ����У��������١����о��ɹ۲쵽��������_____��_____���������з�����Ӧ�Ļ�ѧ����ʽ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����20��ʱ��ij��������(�����ᾧˮ)��ˮ��Һ���������±仯�� ��
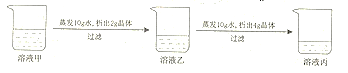
����˵������Ϊ��ȷ�ģ� ��
A. ��Һ���DZ�����Һ
B. 20��ʱ���ù������ʵ��ܽ����40g
C. ��Һ��������10gˮ�������ľ���һ������4g
D. ��Һ�����ʵ���������С����Һ�����ʵ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ȡ6.8g�����ʵ�п�����ձ��У������м���ϡ������ǡ����ȫ��Ӧ������ȥϡ����100g��ʵ������Ƶ��ձ������ʵ�������Ϊ106.6g�������ʲ�����ˮҲ����ϡ���ᷴӦ������㣺
��1����������������Ϊ_____g��
��2������ϡ���������ʵ���������_________����д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼΪA��B��C���ֹ������ʣ�������Ʒˮ����ˮ�е��ܽ�����ߣ���ش��������⣺

��1��P���ʾ�ĺ�����_____��
��2����t2��ʱc�IJ�������Һ��Ϊ���¶��µı�����Һ�����ʵ���������һ��_____��ѡ��������С�����䡱����
��3����A�к�������B����_____�����ᴿA��t3��ʱ����30gA���ʼ��뵽50gˮ�У�����ܽ�����Һ��������_____g��
��4����t1��ʱ��������A��B��C�������ʵı�����Һ������t3���¶ȣ�������Һ�����ʵ����������ɴ�С��˳����_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ķ�������ն��ġ�
����Ʒ���Դȶ���Ϊԭ�ϣ����ӹ����ɵ�ʳƷ��
����һ������Ʒ���Ʊ�
�й��ǴĹ��磬�й��Ŵ��Ͷ��������ö��ഴ���������Ϊ�����Ķ���Ʒ������Ϊ���ֶ���Ʒ����Ҫ�������̣�
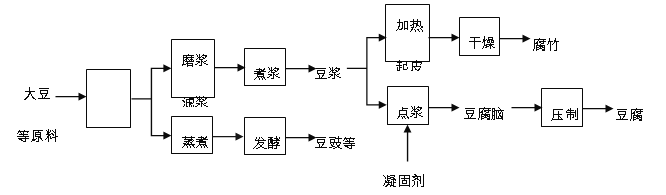
���϶�������Ʒ��Ӫ��
����Ʒ��Ӫ����Ҫ�����ڷḻ�ĵ����ʺ����ϣ� ������иơ��ס����ȿ����ʣ��Լ�ά����B1��B2�ȡ�����Ʒ�и������̴����������̴��벻����֬�����нϺõ���֬���ã��������ܵ͡���ˣ����ֺ�Ѫ֬����Ѫѹ������Ѫ�ܼ������߿ɶ�Զ���Ʒ������Ʒ����Ԥ�����ء��������ɺ���ǿ��������
������������Ʒ�ı�
����Ʒ���зḻ��Ӫ����ɫ��ζ��ѣ���������࣬Ҳ�������彡�������ȣ�����Ʒ���н϶�DZ��谱���ᣬ��������Ӧ������ʳ����Σ�����Ʒ���н϶����ʣ�ʹ�粡��Ӧ��ʳ��ʳ�����⣬θ������ҲӦ������ʳ�ö���Ʒ������̼�θҺ���ڡ��������������ȡ�
��֮������ʳ�ö���Ʒ�����ǿ�ѧ��ʳ֮����
�����������ݻش��������⡣
(1)�ɶ������Ƶö����Ĺ�������__________(����������������ѧ��)�仯��
(2)����Ʒ�к��е����ʡ������ʡ�________��Ӫ�����ʡ�
(3)�ᳫ���ֺ�����Ѫ�ܼ������߶�Զ���Ʒ��������___________��
(4)���ڶ���Ʒ�к���________Ԫ�أ���˿�Ԥ���������ɡ����Ͳ��ȡ�
(5)���й��ڶ���Ʒ��˵����ȷ����_____________(�����)��
A������Ʒ���Զ���Ϊ��Ҫԭ�ϣ��ӹ����ɵ�ʳƷ
B�����������㽬��ѹ�ƺ���Ƴɸ���
C����������Ӧ�þ�����ʳ����Ʒ
D������ƷӪ���ḻ��������˽��ˡ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ȣ�ClO2����һ�ָ�Ч����ȫ����������
��1�����������ƣ�NaClO3�����Ʊ�ClO2��NaClO3����Ԫ�صĻ��ϼ���__��
��2����ҵ������NaClO3�Ļ�ѧ����ʽΪ��2X+2NaCl![]() Cl2��+2NaClO2����X�Ļ�ѧʽΪ__����Ӧǰ�ϼ۷����ı��Ԫ����__��
Cl2��+2NaClO2����X�Ļ�ѧʽΪ__����Ӧǰ�ϼ۷����ı��Ԫ����__��
��3����һ�������£�ClO2���軯�⣨HCN����Ӧ��δ��ƽ������ʾ��ͼ��ͼ��
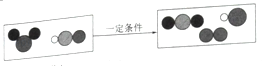
��![]() ����ʾ�����ʵĻ�ѧʽΪ__����Ӧ���ɵ�N2��CO2��������Ϊ__��
����ʾ�����ʵĻ�ѧʽΪ__����Ӧ���ɵ�N2��CO2��������Ϊ__��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com