
| ʵ�鲽�� | ʵ������ | ���ۼ����� |
| ��1��ȡ������ҺA���Թ��У������еμ�������ϡ��� | �����ݲ��� | ����ٲ����� |
| ��2����ȡ������ҺA���Թ��У������еμ��������Ȼ�����Һ�� | ������ɫ���� | ��Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl |
| ��3�������裨2�����õĻ��Һ���ã����ϲ���Һ�еμ���ɫ�ķ�̪��Һ�� | ��Һ�ʺ�ɫ | ����ڳ��� |
���� �����ʵ�顿�����Һ��������Һ�д��ڵ�̼�����ܹ���ϡ���ᷴӦ�������壬���Կɼ���ϡ������Һ���Ƿ�������ð�����ж���Һ�Ƿ���ʣ�NaOH��������ΪNaOH��������ж�����̼��Ӧ����̼���ƺ�ˮ��̼�����е�̼�������һ����ϡ������飮�����һ��̽���Ƿ���ȫ���ʣ������ȼ��������ĸ����ӻ����ӣ���̼���ȫ����ȥ��Ȼ�����÷�̪��Һ�����Ƿ����������ƣ�
����˼�뽻���������������ƹ��屩¶�ڿ�������������ˮ�ֶ����⣬���տ����еĶ�����̼��������˼����
��̽������չ������������Ϣ���н��
��� �⣺�����ʵ�顿��1��̼�����е�̼�������һ����ϡ������飬��������ݲ�������֤���Ѿ����ʣ��ʢٲ�������
��2�������һ��̽���Ƿ���ȫ���ʣ������ȼ��������ĸ����ӣ���̼���ȫ����ȥ��
��3��Ȼ�����÷�̪��Һ�����Ƿ����������ƣ�����а�ɫ�������ɣ���ɫ��̪��Һ����ɫ��֤��NaOH��ȫ�����ʣ���12�֣�
����˼�뽻���������������ƹ��屩¶�ڿ�������������ˮ�ֶ����⣬���տ����еĶ�����̼�����ʣ���ѧ����ʽ��ʾΪ��CO2+2NaOH=Na2CO3+H2O����ˣ���������Ӧ�ܷⱣ�棻
��̽������չ�������������֪�ų���������Ϊ4.4g����CO2��������
�����Ʒ��̼���Ƶ�����Ϊx
Na2CO3+2HCl�T2NaCl+H2O+CO2��
106 44
x 4.4g
$\frac{106}{x}=\frac{44}{4.4g}$
x=10.6g
����ʵ��������Ƶ�������y
CO2+2NaOH=Na2CO3+H2O
80 106
y 10.6g
$\frac{80}{y}=\frac{106}{10.6g}$
y=8g û�б����������Ƶ�������18.6g-10.6g=8g
�ѱ����������Ƶ���������=$\frac{8g}{8g+8g}��$100%=50%
�𰸣�
�����ʵ�顿
| ʵ�鲽�� | ʵ������ | ���ۼ����� |
| ��1��ȡ������ҺA���Թ��У������еμ�������ϡ��� | �����ݲ��� | ����ٲ����� |
| ��2����ȡ������ҺA���Թ��У������еμ��������Ȼ�����Һ�� | ������ɫ���� | ��Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CaCl2=CaCO3��+2NaCl |
| ��3�������裨2�����õĻ��Һ���ã����ϲ���Һ�еμ���ɫ�ķ�̪��Һ�� | ��Һ�ʺ�ɫ | ���� �ڳ��� |
���� ����̽�����������Ƶı���������й�ʵ�鷽������ƺͶ�ʵ�鷽�����������п����ȵ�֮һ�����ڶ�ʵ����Ʒ��������ۣ�Ҫ���������濼�ǣ�һ�Ƿ����Ƿ���У��ܷ�ﵽʵ��Ŀ�ģ�������Ƶķ������бȽϣ����ַ�������㣮��������Ҫ������ʵ�����У�


 ��ʱѵ���������������ϵ�д�
��ʱѵ���������������ϵ�д� �ƸԾ���Ȥζ����ϵ�д�
�ƸԾ���Ȥζ����ϵ�д� ����С����ҵ��ϵ�д�
����С����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
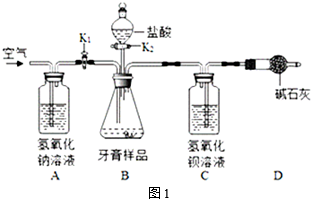
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
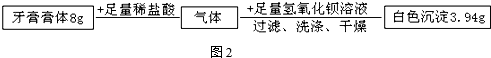
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ȼ��� | B�� | ������ | C�� | ʯ��ˮ | D�� | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ���� | ѡ���Լ� | �������� |
| A | CO2��HCl�� | NaOH��Һ | ������ͨ��ʢ��NaOH��Һ��ϴ��ƿ��ϴ�� |
| B | KNO3��Һ��KOH�� | CuSO4��Һ | ��������CuSO4��Һ�����ˡ������ᾧ |
| C | CaO��CaCO3�� | ϡ���� | �ӹ���ϡ���ᡢ���ˡ�ϴ�ӡ����� |
| D | CuSO4��Һ��H2SO4�� | CuO���� | �ӹ�������ͭ��ĩ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| ��ʵ | ���� | |
| A | �����ͺ�����ѧ�������� | ��ԭ�Ӻͺ�ԭ��������������ͬ |
| B | �Ȼ��ƹ��岻���� | ��Ϊû�д�������� |
| C | ������ˮ���ױ�ѹ�� | �����ȴ�������Ӽ���� |
| D | ���ʵ��������� | ���Ӽ�ļ�����¶ȵĸı���ı� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
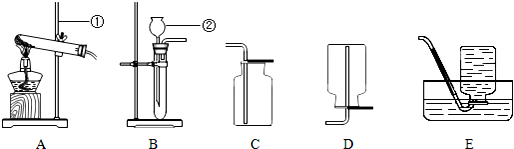
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʱ��������Ż��������Ϲ��� | |
| B�� | �ƾ��Ʋ����������ʪĨ������ | |
| C�� | ��·���·���ʱ�������ô�����ˮ���� | |
| D�� | ͼ��ݵ����ݷ������֣���Һ̬������̼��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ��Ϊ��Ѽ | B�� |  ԣϪ�ڲ� | C�� |  ̫�Ͱ��� | D�� |  ��ɽ�ձ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com