
����Ŀ��Fe(OH)2�ܲ��ȶ���¶���ڿ��������ױ�������Fe(OH)2�������Ļ�ѧ����ʽΪ��________________________________��
Ϊ�˻�ð�ɫ��Fe(OH)2�����������ò���Fe3+��FeSO4��Һ���ò���O2������ˮ���Ƶ�NaOH��Һ��Ӧ�Ʊ���
(1)����������������������FeSO4��Һʱ����û������________��
(2)��ȥ����ˮ���ܽ��O2������________�ķ�����
(3)���ɰ�ɫFe(OH)2�����IJ������ó��ι���ȡ����O2��NaOH��Һ������FeSO4��ҺҺ���£��ټ���NaOH��Һ������������������_________________________________��
(4)����Fe3+���ڵ�����Լ���____________��������______________________________________��
(5)д��Fe��H2O(g)��һ�������·�Ӧ�Ļ�ѧ����ʽ��________________________________��
���𰸡�4Fe(OH)2+O2+2H2O===4Fe(OH)3(3��)
(1)����(1��)
(2)����������(1��)
(3)��ֹ��������NaOH��Һ�ļ��������Һ(2��)
(4)KSCN��Һ(1��) ��Һ��Ϊ��ɫ(1��)
(5)3Fe+4H2O(g)![]() Fe3O4+4H2(2��)
Fe3O4+4H2(2��)
��������Fe(OH)2�ڿ����з����ķ�ӦΪ��4Fe(OH)2+O2+2H2O===4Fe(OH)3���Ʊ�Fe(OH)2�Ĺ���������Ҫ�������Ƿ�ֹFe2+��O2���������������Լ����õ�����ˮ���ü��ȵķ�����ȥ�ܽ������ڵμӷ�Ӧ�Լ�ʱ��Ϊ��ֹ������μӵ�Һ����뷴ӦҺ�У����ѳ��ιܲ���FeSO4��Һ�У�����Fe3+������Լ���KSCN��Һ��Fe3+��SCN��Ӧʹ��Һ��Ϊ��ɫ��


 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ʳ�ξ������������Ӻ���������ɵģ��Ҿ�Ϊ�Ⱦ���Ľ������У���ͼ��ʾ����֪ʳ�ε��ܶ���2.2 g/cm3�������ӵ�����Ϊ6.02��1023/mol����ʳ�ξ�����������������������Ӽ�ľ�����ӽ��� ����(��������)��
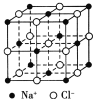
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��Fe2O3+2Al![]() Al2O3+2Fe����������Ӧ������ ���ڸ÷�Ӧ�� Ԫ�صĻ��ϼ����ߣ���Ԫ�ص�ԭ�� ���ӣ��� ���÷�Ӧ�У�Fe2O3������ ��Ӧ��Al������ ��Ӧ�� ���������� �ǻ�ԭ���� ��������� �ǻ�ԭ���
Al2O3+2Fe����������Ӧ������ ���ڸ÷�Ӧ�� Ԫ�صĻ��ϼ����ߣ���Ԫ�ص�ԭ�� ���ӣ��� ���÷�Ӧ�У�Fe2O3������ ��Ӧ��Al������ ��Ӧ�� ���������� �ǻ�ԭ���� ��������� �ǻ�ԭ���
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����18.0 mol��L1��Ũ����ϡ�ͳ�1.80 mol��L1��ϡ����100 mL��ʵ��������£�
(1)�������в�����������д�йصĿո�
������Ͳ��ȡ mL��18.0 mol��L1��Ũ���
���� ���ձ��ڱ�����ע��ʢ������ ���ձ��У�
��������ȴ�����µ�H2SO4��Һ�ز�����ע�� mL������ƿ�У�
������������ˮϴ���ձ�2~3�Σ�����ϴ��ҺҲȫ��ת�Ƶ�����ƿ�У�
������������ƿ�м�����ˮ��ֱ��Һ��ӽ��̶��� mL����
������ ��μ�����ˮ��ʹ��Һ��Һ�����ʹ�ǡ����̶�����ƽ��
���Ǻ�����ƿ���������ߵ���ҡ�ȣ�
������õ�ϡ���ᵹ���Լ�ƿ�У����ñ�ǩ��
(2)���ڲ���������������ʵ������������������Ƶ�H2SO4��Һ�����ʵ���Ũ���к�Ӱ��?(�á�ƫ��ƫС������Ӱ�족��д)
������ƿ������ˮϴ�Ӻ������������ˮ ��
��ҡ�Ⱥ�����Һ��Һ����ڿ̶��ߣ��ּ�ˮ���̶��� ��
(3)����ƿ�ϱ��� (ѡ�����б��)��
���¶� ��Ũ�� ������ ��ѹǿ ���̶���
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�����ᡢ�����̼�������������г��������ʡ������йر�������ȷ����
A����NaHCO3��Һ�м�����������ʵ�����NaOH����Һ�е�������ֻ��![]() ��OH-
��OH-
B��NaHCO3��Һ����c(H+)+ c(H2CO3)= c(OH-)
C��10 mL 0.10 mol/L CH3COOH��Һ��������ʵ�����NaOH����Һ�����ӵ�Ũ���ɴ�С��˳������c(Na+)> c(CH3COO-)> c(OH-)> c(H+)
D���к������pH����ͬ��HCl��Һ��CH3COOH��Һ�����ĵ�NaOH���ʵ�����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���ù�����H2SO4��NaOH��NH3��H2O��NaCl����Һ������ͼ��ʾ����ֿ��������ӣ�����Һ�٢ڢۢ��ֱ���
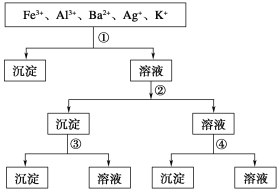
A����NaCl����NaOH����NH3��H2O����H2SO4
B����H2SO4����NaOH����NH3��H2O����NaCl
C����H2SO4����NH3��H2O����NaOH����NaCl
D����NaCl����NH3��H2O����NaOH����H2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����֪����ͼ��ʾ���ʵ��ת����
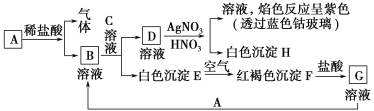
����д���пհף�
(1)д��B�Ļ�ѧʽ________��D�Ļ�ѧʽ________��
(2)д����Eת���F�Ļ�ѧ����ʽ_______________________________��
(3)д�����з�Ӧ�����ӷ���ʽ��
D��Һ��AgNO3��Һ��Ӧ��________________________________________��
��G��Һ�м���A��_____________________________________________��
(4)A��ϡ���ᷴӦ����0.1 mol���壬ת�Ƶ�����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����2015��������A
Share with us
Would you like to have your writing published in this magazine?Then let us know! We pay for stories, anecdotes and jokes:Anecdotes and Jokes��50��What��s made you laugh recently? A funny sign? A colleague��s be haviour? Got a joke? Send it in for Laughter is the Best Medicine!
Email: Juliet@sws.com Smart Animals Up to ��100 Send us a tale about the strange behaviour of unique pets or wildlife in up 300 words.
Email: audry@sws.com Power of Love Up to ��150 Acts of generosity can change lives or just give you that warm feeling full of love. Share your moments 100��500 words.
Email: susan@sws.com My Story ��350 Do you have an inspiring or life-changing story to tell? Your story must be true, unpublished, original and 800-1000words.
Email: nanjc@sws.com For more information, please visit: http://www.sws.com./share.
��1��How much will the magazine pay for a joke to be published?
A. ��50 B. ��100
C. ��150 D. ��350
��2��If you want to share a story of your pets with the readers, you need to submit it to_______.
A. Anecdotes and Jokes B. Smart Animals
C. Power of Love D. My Story
��3��A story showing people��s generosity should be emailed to the editor at_______.
A. juliet@sws.com B. susan@sws.com
C. Audrey@sws.com D. nanjc@sws.com
��4��An inspiring story sent to the magazine should_______.
A. describe strange behaviour
B. contain less than 800 words
C. be real and original
D. be published before
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���ǽ�������A����ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪǿ�ᣬ��ش��������⣺
![]()
(1)��A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ���塣
��D�Ļ�ѧʽ��______��
���ڹ�ҵ�����У������ŷŵ�B���屻��ˮ���պ��γ���______����Ⱦ������
(2)��A�ڳ�����Ϊ�д̼�����ζ�����壬C�Ǻ���ɫ���塣
��A��C�Ļ�ѧʽ�ֱ��ǣ�A______��C______��
��D��Ũ��Һ�ڳ����¿���ͭ��Ӧ������C���壬��д���÷�Ӧ�Ļ�ѧ����ʽ______________________���÷�Ӧ________(����ڡ������ڡ�)������ԭ��Ӧ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com