
����Ŀ����ͼ�ش��������⣺

��1����ij�˳���Ӫ��������Ѫ���е����ʺ������ͣ���������ͼ��ʾ����һ���ֵ�Һ������? [ ]_______��������������_________��
��2��Ŀǰ�ձ���Ϊ��____________��ά���ڻ�����̬����Ҫ���ڻ��ơ�
��3����ij�˻�������ϸ��ƶѪ֢������̬�����仯����ͼ��[ ] ���������ֱ仯�ĸ���ԭ����____________��ֱ��ԭ���Ǹ�ϸ����____________�ṹ�쳣��ʹ����______��������½�����˸ò���Ҫ��Ӱ����������������______�Ρ�
������֯ϸ���е�һ��CO2���ӴӲ�������ɢ��������������Ҫͨ��_______������Ĥ��
��4����ͼʾΪ������ڻ��������ײ��˻���ʱ������ȡ�൱��ͼ��[ ]��Һ�壬�ᷢ��ת��ø�ĺ���ƫ�ߣ�������Ϊ���ײ��˸�ϸ��Ĥ��ͨ��______��
���𰸡�
��1���� ��֯Һ ��֯ˮ��
��2��������Һ��������������
��3���� ��ϸ�� �Ŵ����ʵĸı䣨����ͻ��Ҳ�÷��� Ѫ�쵰�� ���� �� 9
��4���� ����
��������
��1��Ӫ����������Ѫ�������٣���ѹ�½�����֯Һ��������������֯Һ���ӣ�������֯ˮ������
��2��Ŀǰ�ձ���Ϊ������Һ�����ߵ���������ά���ڻ�����̬����Ҫ���ڻ��ơ�
��3��������ϸ��ƶѪ֢���ɻ���ͻ������ģ����ߺ�ϸ������̬�ᷢ���ı䣬����������������������½���Ӱ�����������ĵ����Ρ��ߵȶ���ϸ���ڲ���CO2�ij���Ϊ��֯ϸ���������壬����֯Һ����ëϸѪ�ܣ���Ҫ��Ѫ�����䵽�β����ɺ���ϵͳ�ų����⣬�����ξ�����Ĥ�ǣ�2��������Ĥ��1����֯ϸ��Ĥ��4��ëϸѪ�ܱ�Ĥ��2����ݱڵ�ϸ��Ĥ����9������Ĥ��
��4�����ײ������ڸ�ϸ������ϸ��Ĥͨ��������ϸ���ڵ�ת��ø������Ѫ���ܣ��ʿ���ͨ������Ѫ���ɷ�����ϼ�����


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ȡ0.1 mol/L HA��Һ��0.1 mol/L NaOH��Һ��������(��Ϻ���Һ����ı仯����)����û����Һ��pH=8���Իش��������⣺
��1�������Һ��pH=8��ԭ���� (�����ӷ���ʽ��ʾ)��
��2�������Һ����ˮ�������c(H+) 0.1 mol/L NaOH��Һ����ˮ�������c(H+) (���������������=��)��
��3����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶ�(NH4)2CO3��Һ��pH 7(�������������=��)��
��4������ͬ�¶�����ͬŨ�ȵ���������Һ��A.NH4HCO3��B.NH4A��C.(NH4)2SO4��D.NH4Cl����pH�ɴ�С��˳������ (�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��[2016����]��������ͬ��Ba(OH)2��Һ�У��ֱ�������ʵ���Ũ����ȵ�H2SO4��NaHSO4��Һ���䵼�������������Һ����仯��������ͼ��ʾ��
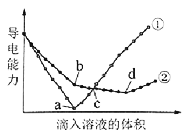
���з�������ȷ����
A���������μ�H2SO4��Һ�ı仯����
B��b�㣬��Һ�д������ڵ�������Na+��OH�C
C��c�㣬����Һ�к�����ͬ����OH�C
D��a��d�����Ӧ����Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb(OH)+��Pb(OH)2��Pb(OH)3��Pb(OH)42������̬��Ũ�ȷ���(��)��pH�仯�Ĺ�ϵ����ͼ��ʾ��
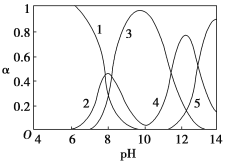
[1��ʾPb2+��2��ʾPb(OH)+��3��ʾPb(OH)2��4��ʾPb(OH)3��5��ʾPb(OH)42]
��1��Pb(NO3)2��Һ�У�![]() ________2(���������������)��������Һ�е����Ȼ����Һ��
________2(���������������)��������Һ�е����Ȼ����Һ��![]() �����ܵ�ԭ����________________________________��
�����ܵ�ԭ����________________________________��
��2����Pb(NO3)2��Һ�е���ϡNaOH��Һ��pH��8ʱ��Һ�д��ڵ�������(Na+����)��______________����pH��8��pH��9ʱ��Ҫ��Ӧ�����ӷ���ʽΪ___________________________________��
��3��ij����С���Ʊ���һ��������Ǧ��������Чȥ��ˮ�е���Ǧ��ʵ�������±���
���� | Pb2+ | Ca2+ | Fe3+ | Mn2+ | Cl |
����ǰŨ��/mg/L | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
������Ũ��/mg/L | 0.004 | 22.6 | 0.040 | 0.053 | 49.8 |
�ϱ��г�Pb2+�⣬����Ǧ�����������ӵ�ȥ��Ч����õ���____________________________��
��4���������Ǧ��(��EH��ʾ)��Ǧ��������Ҫ�����ķ�ӦΪ��2EH(s)+Pb2+![]() E2Pb(s)+2H+������Ǧ�������pH��ΧΪ________(����)��
E2Pb(s)+2H+������Ǧ�������pH��ΧΪ________(����)��
A��3��4 B��6��7 C��8��9 D��10��12
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����������ͼ�У�������Ϊij��Һ�м���ij���ʵ����ʵ�����������Ϊ���ɳ��������ʵ�������ͼ�е���ĸ���������±���
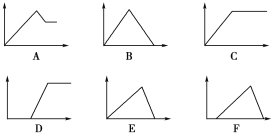
��Һ | ��������� | ��ĸ���� |
������ʯ��ˮ | ͨ����CO2 | |
��AlCl3��Һ | ͨ����NH3 | |
��MgCl2��AlCl3�����Һ | ��μ�NaOH��Һ������ | |
��AlCl3��Һ | ��μ�NaOH��Һ������ | |
��������HCl��AlCl3��Һ | ��μ�NaOH��Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ԭ��غ͵��ض���ʵ���������û����ش��������⣺
��1���ɽ���ѧ��ת��Ϊ������_________��
��2�����з�Ӧ����Ƴ�ԭ��ص��ǣ� ��
�� 2FeCl3+Fe=3FeCl2
�� AlCl3+3NH3��H2O= Al��OH��3��+3NH4Cl
�� NaOH+HCl=NaCl+H2O
��3����FeCl3��Һ��ʴӡˢ��·ͭ������ӷ���ʽΪ�� ��
��4����ͼװ�õ��պϵ��ʱ�������ʾ�е���ͨ������Pt���ĵ缫��Ӧʽ�� �� �����в���0.1 mol����ʱ����������ͭ������ӦΪ________g��
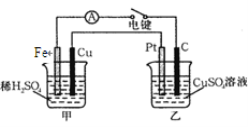
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��(��)��![]() ��
��![]() ��H+��Cu2+��Ba2+��Ag+��Cl��������ѡ���ʵ���������ɵ���ʣ����ö��Ե缫������Һ���е�⡣
��H+��Cu2+��Ba2+��Ag+��Cl��������ѡ���ʵ���������ɵ���ʣ����ö��Ե缫������Һ���е�⡣
��1�������ֱ�ų�H2��O2ʱ������ʵĻ�ѧʽ������_______________________________��
��2�����������������������ų�O2������ʵĻ�ѧʽ������________________________________��
��3�������ֱ�ų����壬�������Ϊ1��1������ʵĻ�ѧʽ������_______________________________��
(��)��֪��Ӧ��Cu+H2SO4(ϡ)===CuSO4+H2��ͨ�����ܷ�������ش�
��4����˵���˷�Ӧ��һ������²��ܷ�Ӧ��ԭ��_______________________________________��
��5������ϡH2SO4��Һ��ͨ���ȵĿ���������ͭ�����ܽ⡣��д���йصĻ�ѧ����ʽ��_______________________________________________________��
��6����������ѧ����֪ʶ���跨ʹ�˷�Ӧ�ܷ���������ķ�����_______________________________����Ӧԭ����______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��Not until all the fish died in the river how serious the pollution was.
A. did the villagers realize
B. the villagers realized
C. the villagers did realize
D. didn��t the villagers realize
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��(2017���������θı�)It seemed that everything the woman owned was in them. This made Hannah very sad, and even more to do something.
A. excited B. determined C. energetic D. grateful
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com