
����Ŀ��ijѧ��Ϊ̽��AgCl�������ܽ��ת�������ʵ�鷽������¼���£�
��������� | ���� |
�������������Ũ�ȵ�AgNO3��Һ��NaCl��Һ��ϵõ���ҺW�����ˣ��õ���ҺX�Ͱ�ɫ����Y |
|
��������ҺX�еμӼ��α���Na2S��Һ | ���ֻ��� |
����ȡ������ɫ����Y���μӼ��α���Na2S��Һ | ������Ϊ��ɫ |
����ȡ������ɫ����Y���μӼ���Ũ��ˮ | �������ܽ� |
��1������������ҺW�д��ڵij����ܽ�ƽ��Ϊ__________________________��
��2���ɲ������Ļ��ǿ��Ʋ⣬��ҺX�г��˺���Na+��NO3�������е�������_________________��
��3����˵���������г�����ڵ����ӷ���ʽΪ_______________������ת������Ҫԭ����______________��
��4����֪��Ag+ + 2NH3�� H2O ![]() Ag(NH3)2 ++ 2H2O����ƽ���ƶ�ԭ�����Ͳ������м���Ũ��ˮ�������ܽ��ԭ��_____________��
Ag(NH3)2 ++ 2H2O����ƽ���ƶ�ԭ�����Ͳ������м���Ũ��ˮ�������ܽ��ԭ��_____________��
��5�����������Ϣ����������Ԥ������ȷ���� ��
A���ڲ�����֮�����μ�Ũ���������AgCl��������
B���ɲ����������Ʋ⣺ʵ���ҿ��ð�ˮϴ��������Ӧ����Թ�
C�����ڰ�ɫ����Y�еμ�NaOH��Һ������Ҳ���ܽ�
���𰸡���1��AgCl(g) ![]() Ag+(aq)+Cl��(aq) ��2��Ag+��Cl��
Ag+(aq)+Cl��(aq) ��2��Ag+��Cl��
��3��2AgCl(s) + S2�� === Ag2S(s) + 2Cl�� ���¶��£�Ag2S��AgCl�ܽ�ȸ�С������������������Ũ�ȵij˻������ܶȻ���
��4��AgCl(g) ![]() Ag+(aq)+Cl��(aq)���������백ˮ��ϣ���������Һ�������ӵ�Ũ�ȣ�ʹ����ƽ�������ƶ�����ʹAgCl�ܽ� ��5��BC
Ag+(aq)+Cl��(aq)���������백ˮ��ϣ���������Һ�������ӵ�Ũ�ȣ�ʹ����ƽ�������ƶ�����ʹAgCl�ܽ� ��5��BC
����������1��AgNO3+NaCl= AgCl+NaNO3�����ڵij����ܽ�ƽ��ΪAgCl(g) ![]() Ag+(aq)+Cl��(aq)��
Ag+(aq)+Cl��(aq)��
��2���ɲ������Ļ�����Ag2S�����Ʋ���ҺX�г��˺���Na+��NO3��������Ag+��Cl����
��3���������г�����ڵ����ӷ���ʽΪ2AgCl(s)+S2��===Ag2S(s)+2Cl��������ת������Ҫԭ�������¶��£�Ag2S��AgCl�ܽ�ȸ�С��
��4����֪��Ag++2NH3��H2O![]() Ag(NH3)2++2H2O��AgCl(g)
Ag(NH3)2++2H2O��AgCl(g)![]() Ag+(aq)+Cl��(aq)���������백ˮ��ϣ���������Һ�������ӵ�Ũ�ȣ�ʹ����ƽ�������ƶ�����ʹAgCl�ܽ���
Ag+(aq)+Cl��(aq)���������백ˮ��ϣ���������Һ�������ӵ�Ũ�ȣ�ʹ����ƽ�������ƶ�����ʹAgCl�ܽ���
��5���ڲ�����֮���������μ�Ũ������NH3�� H2OŨ�Ƚ��ͣ�Ag+ + 2NH3�� H2O ![]() Ag(NH3)2 ++ 2H2O��AgCl(g)
Ag(NH3)2 ++ 2H2O��AgCl(g) ![]() Ag+(aq)+Cl��(aq)�������ƶ�����������AgCl����������B���������백ˮ����Ӧ�������ð�ˮϴ��������Ӧ����Թܣ�C�����ڰ�ɫ����Y�еμ�NaOH��Һ�����������ܽ⡣
Ag+(aq)+Cl��(aq)�������ƶ�����������AgCl����������B���������백ˮ����Ӧ�������ð�ˮϴ��������Ӧ����Թܣ�C�����ڰ�ɫ����Y�еμ�NaOH��Һ�����������ܽ⡣


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ������������Ҫ�Ļ��������ѧ��ѧ������������������������������⣬���������Ρ�FeSO4��7H2O��һ��dz��ɫ���壬�׳��̷��������ڴ����������������ӵķ�ˮ�������̷���ij������ˮ(����ǿ����������![]() )���д�����
)�������
(1)�̷��������������ᷴӦ�����⣬��ҵ�ϻ����ÿ�����ˮ��������(��Ҫ�ɷ�ΪFeS2)����ȡ����֪�÷�Ӧ����������������������һ����ѧ��ѧ������ǿ�ᣬ��д���÷�Ӧ�Ļ�ѧ����ʽ��____________________________��
(2)����ƽ��_______Fe2++_______![]() +_______ ===_______Fe3++_______Cr3++_______H2O
+_______ ===_______Fe3++_______Cr3++_______H2O
(3)���÷�ˮ��������Cr3+�ĺ���Ϊ1��102 mol��m3��������83.4 kg�̷����Դ����÷�ˮ________��(��֪��ˮ���ܶ�Ϊ1 g��cm3)��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���ڿ����ϣ���ʦ��ʾ�˽�������CuSO4��Һ�ķ�Ӧ��ͬѧ�ǹ۲쵽�÷�Ӧ����������ɫ��Cu(OH)2������û�з���ͭ�������ɡ���ijͬѧ�룺��������ɵ�ͭ���ٱ���ɫ���������Ƕ�û�б������أ���������κ�ʵ���Ҽ���̽����ϣ����һ����ʵ������֤�Լ��IJ²��Ƿ���ȷ��
�����ͬѧ���㣬����дһ��ʵ������������ʦ��������ʦ�ṩ�������Ʒ��
(1)ʵ��̽����Ŀ����______________________��
(2)̽�������ݵĻ�ѧԭ����________________(�ñ�Ҫ�����ֺͻ�ѧ����ʽ��ʾ)��
(3)ʵ�����������С��������Ƭ����ֽ��____��____��ʵ��ҩƷ�������ơ�____��____����ͬѧ��̽��ʵ��������ط������ɵ���ɫ�����л��������ĺ�ɫ���������ʹ�õ�ҩƷ��û�����⣬����Ϊ�ú�ɫ��������____________�����ɸú�ɫ�������ԭ����________________________________
(�ñ�Ҫ�����ֺͻ�ѧ����ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�����ᡢ�����̼�������������г��������ʡ������йر�������ȷ����
A����NaHCO3��Һ�м�����������ʵ�����NaOH����Һ�е�������ֻ��![]() ��OH-
��OH-
B��NaHCO3��Һ����c(H+)+ c(H2CO3)= c(OH-)
C��10 mL 0.10 mol/L CH3COOH��Һ��������ʵ�����NaOH����Һ�����ӵ�Ũ���ɴ�С��˳������c(Na+)> c(CH3COO-)> c(OH-)> c(H+)
D���к������pH����ͬ��HCl��Һ��CH3COOH��Һ�����ĵ�NaOH���ʵ�����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��ֻ��һ���Լ����������ȥ���������е�����(������Ϊ����)��д���Լ�����������ơ��������йصĻ�ѧ����ʽ(���ӷ�Ӧ��д���ӷ���ʽ)��
(1)Fe2O3(Al2O3)_________________________________________________��
����ʽ________________________________________________________��
(2)Fe2O3[Fe(OH)3]______________________________________________��
����ʽ_________________________________________________________��
(3)FeSO4��Һ(CuSO4)___________________________________________ __��
����ʽ__________________________________________________________��
(4)FeCl3��Һ(FeCl2)________________________________________________��
����ʽ________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����֪����ͼ��ʾ���ʵ��ת����
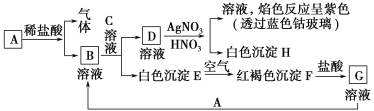
����д���пհף�
(1)д��B�Ļ�ѧʽ________��D�Ļ�ѧʽ________��
(2)д����Eת���F�Ļ�ѧ����ʽ_______________________________��
(3)д�����з�Ӧ�����ӷ���ʽ��
D��Һ��AgNO3��Һ��Ӧ��________________________________________��
��G��Һ�м���A��_____________________________________________��
(4)A��ϡ���ᷴӦ����0.1 mol���壬ת�Ƶ�����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����ҵ�����г�����NaCl��Na2SO4�����ʣ���������ͼ��ʾ��װ�òⶨ��ҵ��������Ч�ɷֵĺ�����
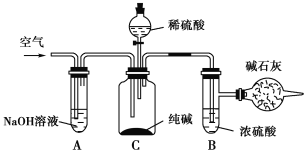
ʵ����̵���Ҫ�����ǣ�
��ȷ��ȡ��������x g(x>2)��������ƿC�С�
��ȷ����װ�м�ʯ�ҵĸ���ܵ�����Ϊy g��
���ӷ�Һ©���л���ע��ϡ���ᣬ�����ٲ�������Ϊֹ��
������������������ӣ�Ȼ�����ȡ�£�ȷ����������ΪW g��
��������ʵ�飬��д���пո�
(1)װ��A��������____________________________���������װ��A���ᵼ��ʵ����ƫ________(���С�����䡱����ͬ)��
(2)װ��B��������__________________���������װ��B���ᵼ��ʵ����ƫ________��
(3)ͨ�������������__________________________________________�������ͨ��������ᵼ��ʵ����ƫ________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��X��Y��Z�������嶼�ܶԴ��������Ⱦ���ڹ�ҵ�϶������ü�Һ���ա���֪X�ǻ�ʯȼ��ȼ�ղ���֮һ�����γ��������Ҫ���ʣ�Y��һ�ֵ��ʣ�����ˮ��Һ����Ư�����ã�Z�����Ṥҵ������β���е��к�����֮һ������ˮ��Ӧ����д���������ʷ�Ӧ�Ļ�ѧ����ʽ��
(1)X��һ����������������Ӧ____________________________________��
(2)Y������������Һ�ķ�Ӧ______________________________________��
(3)Z��ˮ�ķ�Ӧ________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com