
����Ŀ������ת����ϵ�У�A��һ���ճ�������������ܼ���G��������ˮ�Լ��ԣ�Y ��θ�����Ҫ�ɷ֣�K�Dz�����ϡ����İ�ɫ��������Ӧ���ǹ�ҵ��X����Ҫ��Ӧ֮һ��1mol Bͨ����Ӧ�ڵõ�1mol F��B��F����������Ϊ72%��
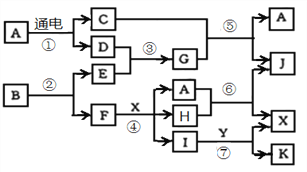
�밴Ҫ����գ�
��1��B��G���еĹ�ͬԪ�������ڱ��е�λ����___________________��
��2����������G��������Ӧ��������һ�ֵ��ʺ�һ���Σ����εĵ���ʽΪ ________________________��
��3��д����Ӧ�ܵ����ӷ���ʽ�����õ����ű������ת�����__________________________��
��4����Ӧ�ݵĻ�ѧ����ʽΪ___________________________________________��
���𰸡� �ڶ�����VA�� 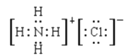
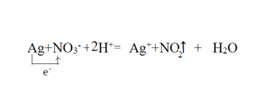

��������A��һ���ճ�������������ܼ���A��ˮ��G��������ˮ�Լ��ԣ�GΪ������Y ��θ�����Ҫ�ɷ֣�YΪ���ᣬC Ϊ������JΪһ�����������Ϸ�Ӧ���ǹ�ҵ���������Ҫ��Ӧ֮һ��A+H��HNO3+NO,����HΪ����������K�Dz�����ϡ����İ�ɫ������KΪ�Ȼ���������IΪ��������YΪ���ᣬFΪ����E Ϊ����������BΪAgN3, ��(Ag)=108��3/(108��3+14��1)=72%,����B��F����������Ϊ72%��������1mol Bͨ����Ӧ�ڵõ�1mol F��
��1��BΪAgN3, GΪ���������еĹ�ͬԪ���ǵ�Ԫ�أ��˵����Ϊ7�������ڱ��е�λ�������ڶ�����VA�壻��ȷ�𰸣��ڶ�����VA�塣
��2��GΪ������������������Ӧ���ɵ������Ȼ�泥��Ȼ��Ϊ���ӻ�����ȴ������Ӽ��ִ��ڹ��ۼ�������ʽΪ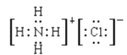 ����ȷ�𰸣�
����ȷ�𰸣�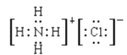 ��
��
��3������ϡ���ᷴӦ������������һ��������ˮ�����ӷ���ʽΪAg+NO3-+2H+=Ag++NO2��+H2O�����ݷ���ʽ����1molAgʧȥ1mol���ӣ���������������е�Ԫ�أ������ű������ת�����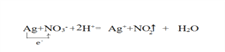 ����ȷ�𰸣�
����ȷ�𰸣�![]() ��
��
��4�������������ڴ��������¼��ȷ�Ӧ����һ��������ˮ����ѧ����ʽΪ ����ȷ�𰸣�
����ȷ�𰸣� ��
��


 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д� Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ�е�Ԫ�ؼ��仯�����ת����ϵ���ش����⣺
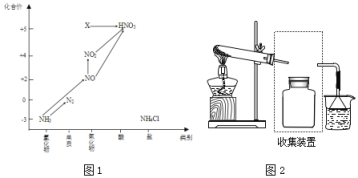
��1��ͼ1�У�X�Ļ�ѧʽΪ___���ӻ��ϼ��Ͽ���X����___�ԣ���������������ԭ������
��2���ش����й���NH3�����⣺
��ʵ���ҳ���NH4Cl��Ca(OH)2��ȡ�������÷�Ӧ�Ļ�ѧ����ʽΪ________��
�������Լ��������ڸ���NH3����___������ĸ����
A��Ũ���� B����ʯ�� C��NaOH����
����Ҫ�ռ�һƿ�������뽫����װ�ò�����������ͼ2����ڻ�������ͼ___��
�ܰ�������Ҫ�Ļ���ԭ�ϣ����Ժϳɶ������ʣ�д����������Ļ�ѧ����ʽ__________________________ ��
��3���ش����й���NO��NO2�����⣺
��������������װ�д�ת�����ɼ���β���Ի�������Ⱦ������β���е��к�����CO��NO��Ӧ��ת��Ϊ�������ŷţ�д����ط�Ӧ�Ļ�ѧ����ʽ��___________________
��NO��NO2��һ��������Ͽ��Ա�NaOH��Һ��ȫ���գ�д����ػ�ѧ����ʽ_____________��
��ҵ���������ð�ˮ����SO2��NO2��ԭ������ͼ��ʾ��
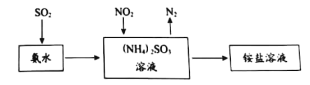
NO2�����չ��̵����ӷ���ʽ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ӡȾ����ֽ���ڶ���ҵ�����Ź㷺��Ӧ�á��о�С����Na2CO3��Һ����SO2�Ʊ�Na2SO3����ʵ���������£�


�������Ͽ�֪����̼������Һͨ���������Ĺ����У���Һ���й���ֵ����������仯����ͼ����ʾ��
��1����ͼ�е���2��ʾ�����Ϊ ���ѧʽ����
��2��ʵ��ʱ������ӦII���м���NaOH��Һ��Ŀ���� ���û�ѧ����ʽ��ʾ����
��3�����ұ��涨��Ʒ��Na2SO3������������97.0%Ϊ�ŵ�Ʒ����93.0%Ϊһ��Ʒ��Ϊ��ȷ��ʵ�����ò�Ʒ�ĵȼ����о�С����������ַ������вⶨ��
������I����ȡ2.570g��Ʒ��������ˮ�ܽ⣬����������˫��ˮʹNa2SO3��ȫ��������Na2SO4���ټ��������BaCl2��Һ�����ó����������ˡ�ϴ�ӡ���������������Ϊ4.660g��ͨ������ȷ����Ʒ��Na2SO3������������д��������̣�
������II����ȡ1.326g��Ʒ�����100mL��Һ��ȡ25.00mL����Һ���μ�0.1250mol/L I2��Һ��ǡ��ʹNa2SO3��ȫ��������Na2SO4ʱ������I2��Һ20.00mL��ͨ������ȷ����Ʒ��Na2SO3������������д��������̣�
���ж�Na2SO3��Ʒ�ĵȼ�����˵�����ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У�����֮��ͨ��һ����Ӧ����ʵ��ͼʾ�仯����
���ʱ�� | ����ת����ϵ | a | b | c | d |
�� |
| NO | NO2 | N2 | HNO3 |
�� | Na2O | Na2O2 | Na | NaOH | |
�� | FeCl2 | FeCl3 | Fe | CuCl2 | |
�� | Al2O3 | NaAlO2 | Al | Al(OH)3 |
A. �٢ڢ� B. �ۢ�
C. �٢ۢ� D. �ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�������� 1mol/L �� NaOH ��Һ240mL��
��1��ʵ�����������ƽ����________g NaOH ���壻
��2������ƿ��ʹ��ǰ�IJ�����________________________
��3����ʵ��ʱ�������������������Һ��Ũ��ƫ�����_________��
�ٳ����������ƹ����ʱ�������
������ƿ������ˮϴ�Ӻ������������ˮ��
����Һδ��ȴ��ת������ƿ��
����ת������������Һ��δϴ���ձ���
������ʱ���ӿ̶��ߡ�
��ҡ�Ⱥ�����Һ��Һ����ڿ̶��ߣ��ּ�ˮ���̶��ߡ�
��4��ȡ���Ƶ� 1mol/L �� NaOH ��Һ10 mL����ϡ�ͳ� 100 m L���ٴ���ȡ�� 10 mL���� 10 mL ��Һ�����ʵ���Ũ��Ϊ________��
��5��ȡ���Ƶ�1mol/L �� NaOH ��Һ10 mL��������������������ַ�Ӧ����Һ������ֲ��䣩���õ�����Һ��Ũ��Ϊ_____________��������Ӧ�����ӷ���ʽΪ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������У��������ʾ����Al(OH)3���������������ʾ�����Լ�����������������в���ȷ����( )
A. 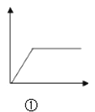 ͼ�ٿɱ�ʾ��NH3��H2O�еμ�AlCl3��Һ������
ͼ�ٿɱ�ʾ��NH3��H2O�еμ�AlCl3��Һ������
B. 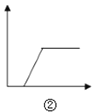 ͼ�ڿɱ�ʾ��NaOH��NaAlO2�Ļ����Һ��ͨ��CO2������
ͼ�ڿɱ�ʾ��NaOH��NaAlO2�Ļ����Һ��ͨ��CO2������
C. 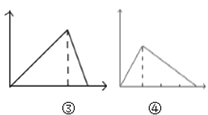 ͼ�ۿɱ�ʾ��NaOH��NaAlO2�Ļ��Һ�еμ�HCl��Һ����������ͼ�ܿɱ�ʾ�������ữ��AlCl3��Һ�еμ�NaOH������
ͼ�ۿɱ�ʾ��NaOH��NaAlO2�Ļ��Һ�еμ�HCl��Һ����������ͼ�ܿɱ�ʾ�������ữ��AlCl3��Һ�еμ�NaOH������
D. 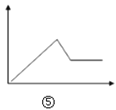 ͼ�ݿɱ�ʾ��MgCl2��AlCl3�Ļ��Һ�еμ�NaOH��Һ������
ͼ�ݿɱ�ʾ��MgCl2��AlCl3�Ļ��Һ�еμ�NaOH��Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С���о���������Al2O3����������֪����������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2����������������ȡAl2O3�Ĺ���������
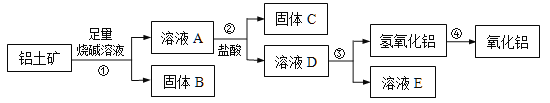
��1������B����Ҫ��;����д��1��������________________��
��2�������������������м��������ռ���Һ��������Ӧ�����ӷ���ʽ�� �� ��
�������У��������������Ļ�ѧ����ʽ�� ��
��3����ʵ����������ù��徫ȷ����������С�鷢�������������������������ԭ������������ȣ������������Al2O3������������_______��������һλС����
��4����ҵ����ȡAlCl3��Al2O3��C��Cl2�ڸ��������·�Ӧ��ÿ����0��5 mol ̼���ʣ�ת��1 mol���ӣ���Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������仯ʾ��ͼ�������Ȼ�ѧ����ʽ��ȷ����( )
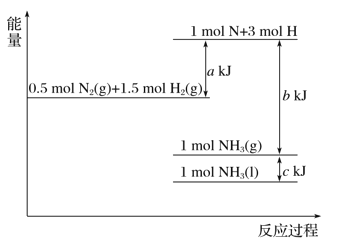
A. N2(g)��3H2(g)===2NH3(g) ��H����(b��a) kJ��mol��1
B. N2(g)��3H2(g)===2NH3(g) ��H����(a��b) kJ��mol��1
C. 2NH3(l)===N2(g)��3H2(g) ��H��2(a��b��c) kJ��mol��1
D. 2NH3(l)===N2(g)��3H2(g) ��H��2(b��c��a) kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��WΪ���ֶ���������Ԫ�أ�X��Y�����ڱ��е����λ����ͼ��ʾ��Xn-��Ym+��Z+������ͬ�ĵ��Ӳ�ṹ��W�����ڲ������������������֮�͵��ڴ���������������˵����ȷ����
![]()
A. ԭ�Ӱ뾶:r(X)>r(Y)>r(Z)>r(W)
B. X�γɵ���������������Ϊ4��
C. ����������Ӧˮ����ļ���:Z<Y
D. Y��Z��W��Ӧ������������ˮ����֮���ܹ��������Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com