
| ��һ�� | �ڶ��� | ������ | |
| ��Ʒ������/g | 7.54 | 15.08 | 35.00 |
| ������������/L | 0.672 | 1.344 | 2.688 |
| �������/g | 0.80 | 1.60 | 3.20 |
| 2.688L |
| 22.4L/mol |
| n |
| V |
| 2.688L |
| 22.4L/mol |
| n |
| V |
| 0.12mol |
| 0.03L |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
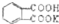 �ⶨNaOH��Һ��Ũ�ȣ���ÿ�ζ���ȷȡ���ڱ����������Ϊ0.2040g���ζ��յ�ʱ��Һ��pHԼΪ9.1��pH�Ʋ��������ζ�ʱ�÷�̪��ָʾ�����ô���NaOH��Һ�ζ��ڱ���������أ�������������ʵ�飬ÿ����������������Һ��������±���
�ⶨNaOH��Һ��Ũ�ȣ���ÿ�ζ���ȷȡ���ڱ����������Ϊ0.2040g���ζ��յ�ʱ��Һ��pHԼΪ9.1��pH�Ʋ��������ζ�ʱ�÷�̪��ָʾ�����ô���NaOH��Һ�ζ��ڱ���������أ�������������ʵ�飬ÿ����������������Һ��������±���| ʵ���� | �ڱ����������������g�� | ����NaOH��Һ�����mL�� |
| l | 0.2040 | 23.20 |
| 2 | 0.2040 | 19.95 |
| 3 | 0.2040 | 20.05 |
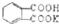 ��ʽ��Ϊ204.0����1mol�ڱ��������������1mol NaOH��Ӧ��
��ʽ��Ϊ204.0����1mol�ڱ��������������1mol NaOH��Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������κ�ˮ�ķ�Ӧһ�����кͷ�Ӧ |
| B�����ֽⷴӦһ��û�е��ʲμ� |
| C������һ�ֵ��ʺ�һ�ֻ�����ķ�Ӧһ�����û���Ӧ |
| D��������֮��һ�����ܷ�����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
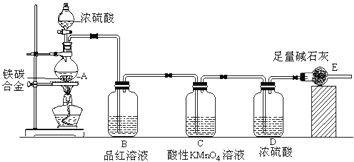
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

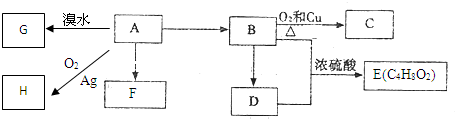
| A�������顢�⾧�����ϩ������ʹ��ˮ��ɫ |
| B���⾧�����ϩ��Ϊͬ���칹�� |
| C��������Ķ��ȴ��ﹲ��3�� |
| D�������顢�⾧����Cԭ�Ӷ��γ�4��������������Ƕ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
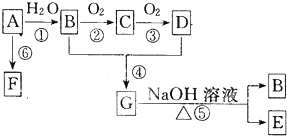
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com