
Ϊ�ⶨijƷ��ϴ������(����)������ijѧ��������֪���ʵ���Ũ�ȵ�NaOH��Һ���ⶨ��ϴ��(����)�����ʵ���Ũ��ʱ��ѡ���̪��ָʾ��������д���пհף�
��1����ʽ�ζ��ܵ�ʹ�÷�������ȷ������Ⱥ�˳��Ϊ����ѡ����ţ���
A��������������Һ��ϴ��������������������Һ
B���ų�����������Һ���еζ�
C��������ʼ����
D����©����ˮϴ2-3��
��2���ñ���NaOH��Һ�ζ����������ʱ,���ֿ��Ƽ�ʽ�ζ��ܵIJ���������ҡ����ƿ���۾�Ӧע�� ��
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ������ͼ��ʾ��������NaOH��Һ�����Ϊ mL��
��4��ijѧ����������ʵ��ֱ��¼�й��������±���
| �ζ����� | ������������/mL | 0.1000 mol/LNaOH��Һ�����/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 2.00 | 28.15 | 26.15 |
| �ڶ��� | 25.00 | 1.50 | 29.50 | 28.00 |
| ������ | 25.00 | 0.20 | 26.55 | 26.35 |
(1) DACB��
��2����ƿ����Һ��ɫ�ı仯��
��3��26.10mL��
��4�� c(HCl)��0.105 mol/L��
��5��ƫ��ƫ��
���������������1���������裺�����������ϴ������װҺ��������ʼ�������к͵ζ����յ��жϡ����㡢���ݴ�����
��2���ζ�ʱ�����ֿ��ƻ��������ְ�˳ʱ��ҡ����ƿ���۾��۲���ƿ����Һ��ɫ�ı仯��
��3���۲����ʱҪƽ�ӣ�
��4��c(HCl)��0.1000*��26.35+26.15��/2/25.00="0.105" mol/L��
��5��ȡ����Һʱ�����Ӻ��ӣ���ʹ����Һ�Ķ���ƫС ��ʹ����ϴ������(����)��Ũ��ƫ�����ζ����������ݣ���ʹ��Һ�������ƫ�Ӷ�ʹ����ϴ������(����)��Ũ��ƫ��
���㣺�������顣


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D������������ˮ����ȫ���룬��������������±���
| ������ | Na+��Al3+��Ba2+��H+��NH4+ |
| ������ | SO42-��OH-��CO32-��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ��Ϊ�˲ⶨ��ͭ����Ҫ�ɷ���Cu2S����Cu2S�������������������ͼװ�á�ʵ��ʱ�����²��������
| A������ȫ��������ʹ���Ϊ��ͼװ�ã������װ�õ������ԡ� |
| B����ȡ��ϸ�Ļ�ͭ����Ʒ1.000g�� |
| C���������õ���ƷС�ĵط���Ӳ�ʲ������С� |
| D����ÿ����1L�����ʹ�������� |
| �ζ� ���� | ������Һ�� ���/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.04 | 21.03 |
| 2 | 25.00 | 1.98 | 21.99 |
| 3 | 25.00 | 3.20 | 21.24 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij�о���ѧϰС��Ϊ��̽������ĵ������������������ʵ�顣
ʵ��һ�����Ʋ��궨������Һ��Ũ��
ȡ����������250 mL 0.2 mol��L��1�Ĵ�����Һ����0.2 mol��L��1�Ĵ�����Һϡ�ͳ�����Ũ�ȵ���Һ������NaOH����Һ�����������Һ��Ũ�Ƚ��б궨���ش��������⣺
(1)����250 mL 0.2 mol��L��1������Һʱ��Ҫ�õ��IJ�����������Ͳ���ձ����������� �� ��
(2)Ϊ�궨ij������Һ��ȷŨ�ȣ���0.200 0 mol��L��1��NaOH��Һ��20.00 mL������Һ���еζ������εζ�����NaOH��Һ��������£�
| ʵ����� | 1 | 2 | 3 | 4 |
| ����NaOH��Һ�����(mL) | 20.05 | 20.00 | 18.80 | 19.95 |
| ����Ũ��(mol��L��1) | 0.001 0 | 0.010 0 | 0.020 0 | 0.100 0 | 0.200 0 |
| pH | 3.88 | 3.38 | 3.23 | 2.88 | 2.73 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������NaOH��������0.1 000mol��L-1NaOH��Һ500mL��
��1����������ƽ��ȡNaOH����________g����Һ���ƹ����õ����в������������״�ʹ�õ��Ⱥ�˳��������________ (������ѡ�����)
A�������� B����ͷ�ι� C���ձ� D��500mL����ƿ
��2���������Ƶ�0.1000mol��L-1NaOH��Һͨ���к͵ζ��ⶨһԪ����HA��ҺŨ�ȣ�ÿ�εζ�ȡ�õ�HA��Һ��Ϊ20.00mL��ʹ�÷�̪��ҺΪָʾ�����ζ��յ�ı�־��____________________________���ζ���ʵ�����ݼ�¼��
| �ζ����� | NaOH��Һ�����mL�� | |
| V1 | V2 | |
| 1 | 3.05 | 44 |
| 2 | 1.45 | 41.5 |
| 3 | 7.65 | 47.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һ��
������ɷֽ�Ϊ���¼�����
a. ��ȡ20.00mL�����NaOH��Һע��ྻ����ƿ��������2-3�η�̪
b. �ñ�������Һ��ϴ�ζ���2-3��
c. ��ʢ�б���Һ����ʽ�ζ��̶ܹ��ã�����Һ��ʹ�ζ��ܼ��������Һ
d. ȡ��������Һע����ʽ�ζ�����0�̶�����2-3cm
e. ����Һ����0��0�̶����£����¶���
f. ����ƿ���ڵζ��ܵ����棬�ñ�������Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
���������գ�
��1����ȷ������˳���ǣ��������ĸ��д��__________________ ____��
��2���ζ��յ�ʱ��Һ����ɫ�仯�� ��
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���_____ ___��
| A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������� |
| B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и��� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��� |
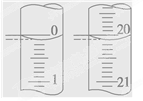
| �ζ� ���� | ����NaOH��Һ�����/mL | 0.1000mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.20 | 20.22 | |
| �ڶ��� | 25.00 | 0.56 | 24.54 | |
| ������ | 25.00 | 0.42 | 20.40 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ�Ȼ������л��������ô����������Ϊ��ɫ���壬178��ʱ���������׳��⣬��ˮ��ᷢ�Ȳ�����������ʵ����������װ���Ʊ�������ˮ�Ȼ������䷴Ӧԭ��Ϊ��2Al + 6HCl(g) �� 2AlCl3 + 3H2��
���������գ�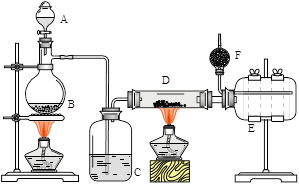
��1��д��B����ƿ�з�����Ӧ�Ļ�ѧ����ʽ��_______________________________________��
��2��C��ʢ�е��Լ�Ϊ__________������ʵ��ʱӦ�ȵ�ȼ_____��ѡ�B����D�������ƾ��ơ�
��3�� �ô̵ֶ�������D��E��Ŀ����_________��ѡ����ţ���
a. ������ b. ������ c. ƽ����ѹ d. ��������
Eƿ��������_______________________________________________________��
��4��F��ʢ�м�ʯ�ң���Ŀ����_________��ѡ����ţ���
a. ����HCl b. ����Cl2 c. ����CO2 d. ����H2O
��5���ٽ�D�й����Ϊ�����Ȼ�����AlCl3��6H2O��Ҳ�ܽ�����ˮ�Ȼ������Ʊ�����ʱͨ��HCl�����Ŀ����_____________________________________________��
����ʵ���������Ʋ��������յõ��������Ǽ�ʽ�Ȼ���[��ѧʽΪAl2(OH)nCl(6-n)]����������ԭ�����Ȼ�����40%���������n��ֵΪ_______��
��6�����˽��齫����װ��A��B�е��Լ���ΪŨ����Ͷ������̣�����װ�ú��Լ������䣬Ҳ���Ʊ���ˮAlCl3����ʵ֤���������Ƚ�Σ�գ���������� _________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ijԭ���װ������ͼ��ʾ������ܷ�ӦΪ2Ag��Cl2��2AgCl������˵����ȷ����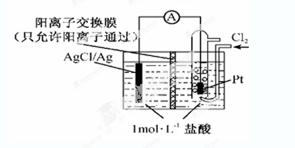
| A��������ӦΪAgCl ��e����Ag ��Cl�� |
| B���ŵ�ʱ������Ĥ�Ҳ���Һ���д�����ɫ�������� |
| C������NaCl��Һ�������ᣬ�����ܷ�Ӧ��֮�ı� |
| D������·��ת��0.01 mol e��ʱ������Ĥ�����Һ��Լ����0.02 mol���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ϲ����������ԭ��صĻ�ѧ��Ӧ��
| A��2FeCl3(aq)��2KI(aq) = 2FeCl2(aq)��2KCl(aq)��I2(aq) ��H <0 |
| B��Ba(OH)2��8H2O(s)+2NH4Cl(s) = BaCl2(aq)+2NH3��H2O(aq)+8H2O��1�� ��H >0 |
| C��4Al(s)+ 6H2O��1��+ 3O2(g)==4Al(OH)3(s) ��H <0 |
| D��Zn(s)+2MnO2(s)+2H2O��1�� = 2MnOOH(s) +Zn(OH)2(s) ��H <0 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com