
ЁОЬтФПЁПЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉГЃЮТЯТЃЌНЋm molЁЄLЃ1ЕФCH3COOHШмвККЭn molЁЄLЃ1NaOHШмвКЕШЬхЛ§ЛьКЯКѓЃЌШмвКЕФpHЃН7ЃЌдђmгыnЕФДѓаЁЙиЯЕЪЧm____nЃЈЬюЁАЃОЁБЁЂЁАЃМЁБЛђЁА=ЁБЃЌЯТЭЌЃЉЃЌдЫсжаc(H+)гыМюжаc(OH-)ЕФДѓаЁЙиЯЕЪЧc(H+)____c(OH-)ЁЃ
ЃЈ2ЃЉГЃЮТЯТЃЌНЋХЈЖШОљЮЊ0.1molЁЄLЃ1ЕФCH3COONaЁЂCH3COOHШмвКЕШЬхЛ§ЛьКЯКѓЃЌШмвКГЪЫсадЁЃдђЛьКЯШмвКжаИїРызгХЈЖШгЩДѓЕНаЁХХађЮЊ_____ЁЃЙигкИУЛьКЯШмвКЕФЫЕЗЈВЛе§ШЗЕФЪЧ___(ЬюДњКХ)ЁЃ
aЃЎДЫШмвКвЛЖЈгаc(Na+)+c(H+)=c(OHЃ)+c(CH3COOЃ)
bЃЎДЫШмвКвЛЖЈгаc(Na+)=c(CH3COOH)+c(CH3COOЃ)
cЃЎДЫШмвКжаЫЎЕФЕчРыГЬЖШвЛЖЈДѓгкДПЫЎЕФЕчРыГЬЖШ
dЃЎЯђДЫШмвКжаМгЩйСПЧтбѕЛЏФЦЛђбЮЫсЃЌШмвКpHБфЛЏВЛДѓ
ЃЈ3ЃЉгУ0.1000 molЁЄLЃ1NaOHШмвКЕЮЖЈ20.00mLФГХЈЖШЕФCH3COOHШмвКЃЌЕЮЖЈЧњЯпШчгвЭМЫљЪОЁЃЦфжаЕуЂйЫљЪОШмвКжаc(CH3COO-)=1.7c(CH3COOH)ЃЌЕуЂлЫљЪОШмвКжаc(CH3COO-)+c(CH3COOH)=c(Na+)ЁЃСаЪНВЂМЦЫуДзЫсЕФЕчРыГЃЪ§_____ЃЌCH3COOHЕФЮяжЪЕФСПХЈЖШЮЊ____ molЁЄLЃ1ЁЃ
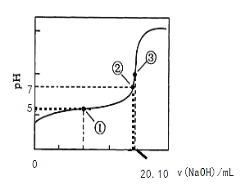
ЁОД№АИЁПЃО ЃМ c(CH3COO- )ЃОc(Na+)ЃОc(H+)ЃОc(OH-) bc 1.7ЁС10-5 0.1005
ЁОНтЮіЁП
ЃЈ1ЃЉpHЃН7ЃЌЫЕУїc(H+)=c(OH-)ЃЌCH3COOHЮЊШѕЫсЃЌЧтбѕЛЏФЦЪЧЧПМюЃЌвђДЫЗДгІКѓДзЫсЪЧЙ§СПЕФЃЌвђЖјmЃОnЃЌc(H+)ЃМc(OH-)ЃЛ
ЃЈ2ЃЉНЋХЈЖШОљЮЊ0.1molЁЄLЃ1ЕФCH3COONaЁЂCH3COOHШмвКЕШЬхЛ§ЛьКЯc(CH3COO-)зюЖрЃЌХЈЖШзюДѓЃЌЧвгаc(CH3COO-)ЃОc(Na+)ЃЌШмвКГЪЫсадЃЌгаc(H+)ЃОc(OH-)ЃЌФЧУДЛьКЯШмвКжаИїРызгХЈЖШгЩДѓЕНаЁХХађЮЊЃКc(CH3COO-)ЃОc(Na+)ЃОc(H+)ЃОc(OH-)ЃЛ
aЃЎИљОнЕчКЩЪиКуПЩжЊc(Na+)+c(H+)=c(OHЃ)+c(CH3COOЃ)ЃЌaЯюе§ШЗЃЛ
bЃЎИљОнЮяСЯЪиКуга2c(Na+)=c(CH3COOH)+c(CH3COOЃ)ЃЌbЯюДэЮѓЃЛ
cЃЎЛьКЯШмвКГЪЫсадЃЌвжжЦСЫШмвКжаЫЎЕФЕчРыЃЌвђДЫШмвКжаЫЎЕФЕчРыГЬЖШаЁгкДПЫЎЕФЕчРыГЬЖШЃЌcЯюДэЮѓЃЛ
dЃЎаЮГЩЕФЛьКЯШмвКЮЊЛКГхШмвКЃЌМгШыЧтбѕЛЏФЦвжжЦСЫЫЎНтДйНјСЫЕчРыЃЌМгШыбЮЫсвжжЦСЫЕчРыЃЌДйНјСЫЫЎНтЃЌвђДЫШмвКpHБфЛЏВЛДѓЃЌdЯюе§ШЗЃЛ
Д№АИбЁbcЁЃ
ЃЈ3ЃЉЕуЂйШмвКжаc(CH3COO-)=1.7c(CH3COOH)ЃЌДЫЪБpH=5ЃЌc(H+)=10-5mol/LЃЌДзЫсЕФЕчРыГЃЪ§Ka=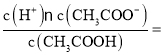 1.7ЁС10-5ЃЛ
1.7ЁС10-5ЃЛ
ЕуЂлШмвКжаc(CH3COO-)+c(CH3COOH)=c(Na+)ЃЌЫЕУїNaOHШмвКгыCH3COOHЧЁКУЭъШЋЗДгІЃЌдђДЫЪБc(CH3COOH)=![]() =0.1005mol/LЁЃ
=0.1005mol/LЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМюЪНЬМЫсюм[Cox(OH)y(CO3)z]ГЃгУзїЕчзгВФСЯЃЌДХадВФСЯЕФЬэМгМСЃЌЪмШШЪБПЩЗжНтЩњГЩШ§жжбѕЛЏЮяЁЃЮЊСЫШЗЖЈЦфзщГЩЃЌФГЛЏбЇаЫШЄаЁзщЭЌбЇЩшМЦСЫШчЭМЫљЪОзАжУНјааЪЕбщЁЃ
ЃЈ1ЃЉЧыЭъГЩЯТСаЪЕбщВНжшЃК
ЂйГЦШЁ3.65gбљЦЗжУгкгВжЪВЃСЇЙмФкЃЌГЦСПввЁЂБћзАжУЕФжЪСПЃЛ
ЂкАДШчЭМЫљЪОзАжУзщзАКУвЧЦїЃЌВЂМьбщзАжУЦјУмадЃЛ
ЂлМгШШМзжаВЃСЇЙмЃЌЕБввзАжУжа________________________ЃЈЬюЪЕбщЯжЯѓЃЉЃЌЭЃжЙМгШШЃЛ
ЂмДђПЊЛюШћaЃЌЛКЛКЭЈШыПеЦјЪ§ЗжжгКѓЃЌГЦСПввЁЂБћзАжУЕФжЪСПЃЛЂнМЦЫуЁЃ
ЃЈ2ЃЉВНжшЂмжаЛКЛКЭЈШыПеЦјЪ§ЗжжгЕФФПЕФЪЧ_________________________________________ЁЃ
ЃЈ3ЃЉФГЭЌбЇШЯЮЊЩЯЪіЪЕбщзАжУжаДцдквЛИіУїЯдШБЯнЃЌЮЊНтОіетвЛЮЪЬтЃЌПЩбЁгУЯТСазАжУжаЕФ____ЃЈЬюзжФИЃЉСЌНгдкзАжУ______жЎЧАЃЈЬюЁАМзЁБЛђЁАввЁБЛђЁАБћЁБЛђЁАЖЁЁБЃЉЁЃ
ЃЈ4ЃЉШєАДе§ШЗзАжУНјааЪЕбщЃЌВтЕУШчЯТЪ§ОнЃКдђИУМюЪНЬМЫсюмЕФЛЏбЇЪНЮЊ______________ЁЃ
ввзАжУЕФжЪСП/g | БћзАжУЕФжЪСП/g | |
МгШШЧА | 80.00 | 62.00 |
МгШШКѓ | 80.36 | 62.88 |
ЃЈ5ЃЉCoCl2ЁЄ6H2OГЃгУзїЖрВЪЫЎФрЕФЬэМгМСЃЌвдКЌюмЗЯСЯЃЈКЌЩйСПFeЁЂAlЕШдгжЪЃЉжЦШЁCoCl2ЁЄ6H2OЕФвЛжжЙЄвеШчЯТЃК
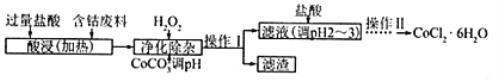
вбжЊЃК
ГСЕэЮя | Fe(OH)3 | Fe(OH)2 | Co(OH)2 | Al(OH)3 |
ПЊЪМГСЕэ(pH) | 2.3 | 7.5 | 7.6 | 3.4 |
ЭъШЋГСЕэЃЈpHЃЉ | 4.1 | 9.7 | 9.2 | 5.2 |
ЂйОЛЛЏГ§дгжЪЪБЃЌМгШыH2O2ЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ__________________________________ЁЃ
ЂкМгШыCoCO3ЕїpHГ§дгЕУЕНТЫдќAl(OH)3ЁЂFe(OH)3ЃЌдђpHгІЕїНкжС_____ЁЃЃЈЬюpHжЕЕФЗЖЮЇЃЉ
ЂлМгбЮЫсЕїећpHЮЊ2ЁЋ3ЕФФПЕФЮЊ_____________________________________ЁЃ
ЂмВйзїЂђЙ§ГЬЮЊ_________________________________ЃЈЬюВйзїУћГЦЃЉЁЂЙ§ТЫЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЙигкгаЛњЛЏКЯЮяЕФЫЕЗЈе§ШЗЕФЪЧ
A.УКжаКЌгаБНЁЂМзБНЁЂЖўМзБНЕШЗМЯуЬўЃЌПЩЭЈЙ§ИЩСѓЕУЕН
B.C4H8Cl2ЕФЭЌЗжвьЙЙЬхга9жж(ВЛКЌСЂЬхвьЙЙ)
C.БНввЯЉ(![]() )ЗжзгжаЫљгадзгВЛПЩФмЙВЦНУц
)ЗжзгжаЫљгадзгВЛПЩФмЙВЦНУц
D.ввДМКЭввЫсОљФмгыЫсадKMnO4ШмвКЗЂЩњбѕЛЏЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПТБЫиЕЅжЪX2(XБэЪОЮЊClЁЂ Br)гыH2ЗДгІФмСПзЊЛЏЙиЯЕШчЯТЃЌЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ
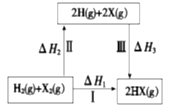
A.Й§ГЬ II ЮќЪеФмСПЃЌЙ§ГЬ III ЗХГіФмСП
B.Cl2(g)ЃЋ2HBr(g)=Br2(g)ЃЋ2HCl(g) ИУЗДгІФмздЗЂНјааЃЌдђЁїGЉ0
C.ЭООЖIЩњГЩHClЪБЗХГіЕФШШСПБШЩњГЩHBrЪБЕФЖрЃЌЫЕУїЩњГЩHClБШЩњГЩHBrШШСІбЇЩЯЧїЪЦИќДѓ
D.ЩњГЩHXЕФЗДгІШШгыЭООЖЮоЙиЃЌЁїH1 = ЁїH2 +ЁїH3
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП25ЁцЪБЃЌЯђ500 mL0.2 mol/L NaOHШмвКжаЭЈШыSO2ЦјЬх(КіТдЗДгІЙ§ГЬжаШмвКЕФЬхЛ§БфЛЏ)ЃЌЩшЗДгІЙ§ГЬжа![]() =xЃЌвбжЊH2SO3ЕФЕчРыЦНКтГЃЪ§ЃКKa1=1.2ЁС10-2ЃЌKa2=5.6ЁС10-8ЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
=xЃЌвбжЊH2SO3ЕФЕчРыЦНКтГЃЪ§ЃКKa1=1.2ЁС10-2ЃЌKa2=5.6ЁС10-8ЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
A. x=1ЪБЃЌc(SO32-)+c(HSO3-)=0.2 mol/L
B. x<1ЪБЃЌШмвКПЩФмЮЊЫсад
C. x=![]() ЪБЃЌc(SO32-)+c(HSO3-)+c(OH-)=c(Na+)+c(H+)
ЪБЃЌc(SO32-)+c(HSO3-)+c(OH-)=c(Na+)+c(H+)
D. x<![]() ЪБЃЌЫцЭЈШыSO2СПдіДѓЃЌ
ЪБЃЌЫцЭЈШыSO2СПдіДѓЃЌ![]() ж№НЅМѕаЁ
ж№НЅМѕаЁ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПУРЁЂЕТСНЙњГЩЙІКЯГЩОпгаЛЏбЇЬиадЕФЧтТСЛЏКЯЮя![]() ЃЌЙигкЧтТСЛЏКЯЮяЕФЭЦВтВЛе§ШЗЕФЪЧЃЈ ЃЉ
ЃЌЙигкЧтТСЛЏКЯЮяЕФЭЦВтВЛе§ШЗЕФЪЧЃЈ ЃЉ
A.ЧтТСЛЏКЯЮяжаТСЯд![]() МлЃЌЧтЯд
МлЃЌЧтЯд![]() Мл
Мл
B.ЧтТСЛЏКЯЮягыЫЎЗДгІЩњГЩЧтбѕЛЏТСКЭЧтЦј
C.ТСгыЫЎеєЦјИпЮТЯТЗДгІЩњГЩЧтТСЛЏКЯЮя
D.ЧтТСЛЏКЯЮяОпгаЧПЛЙдад
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП[ЛЏбЇЁЊбЁао3ЃЛЮяжЪНсЙЙгыаджЪ]
УїГЏЁЖЬьЙЄПЊЮяЁЗжагаЪРНчЩЯзюдчЕФЙигкСЖаПММЪѕЕФМЧдиЃЌаПвВЪЧШЫЬхБиашЕФЮЂСПдЊЫиЁЃЛиД№ЯТСаЮЪЬтЃК
(1)ЛљЬЌZnдзгКЫЭтЕФзюИпФмВуЗћКХЪЧ________ЃЌЛљЬЌZn2+зюЭтВуЕчзгХХВМЪНЮЊ________ЁЃ
(2)СђЫсаПШмгкАБЫЎаЮГЩ[Zn(NH3)4]SO4ШмвКЁЃ
ЂйзщГЩ[Zn(NH3)4]SO4ЕФдЊЫижаЃЌГ§ZnЭтЦфгрдЊЫиЕФЕчИКадгЩДѓЕНаЁХХађЮЊ________ЁЃ
ЂкЯђ[Zn(NH3)4]SO4ШмвКжаж№ЕЮЕЮМгNaOHШмвКЃЌЮДГіЯжЛызЧЃЌЦфдвђЪЧ________ЁЃ
ЂлвбжЊ[Zn(NH3)4]2+ЕФПеМфЙЙаЭгы![]() ЯрЭЌЃЌдђдк[Zn(NH3)4]2+жаZn2+ЕФдгЛЏРраЭЮЊ________ЃЌNH3взвКЛЏЕФдвђЪЧ________________________________ЁЃ
ЯрЭЌЃЌдђдк[Zn(NH3)4]2+жаZn2+ЕФдгЛЏРраЭЮЊ________ЃЌNH3взвКЛЏЕФдвђЪЧ________________________________ЁЃ
Ђмдк[Zn(NH3)4]SO4ОЇЬхжаДцдкЕФзїгУСІга________ЁЃ
AЃЎРызгМќ BЃЎМЋадЙВМлМќ CЃЎЧтМќ
DЃЎХфЮЛМќ EЃЎЗЖЕТЛЊСІ FЃЎН№ЪєМќ
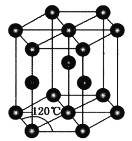
(3)ZnOгыZnSНсЙЙЯрЫЦЃЌZnOЕФШлЕуЮЊ1975ЁцЃЌZnSЕФШлЕудМЮЊ1700ЁцЁЃZnOШлЕуБШZnSИпЕФдвђЪЧ________________________________ЁЃ
(4)ГЃЮТЯТН№ЪєаПОЇЬхЕФОЇАћЮЊСљЗНзюУмЖбЛ§(ШчЭМЫљЪО)ЃЌШєаПдзгЕФАыОЖЮЊrnmЃЌСљРтжљЕФИпЮЊ![]() ЃЌдђН№ЪєаПОЇЬхЕФПеМфРћгУТЪЪЧ________(гУКЌІаЕФДњЪ§ЪНБэЪО)ЁЃ
ЃЌдђН№ЪєаПОЇЬхЕФПеМфРћгУТЪЪЧ________(гУКЌІаЕФДњЪ§ЪНБэЪО)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЫЕЗЈжае§ШЗЕФЪЧ![]()
![]()
A.ЬьШЛЦјЕФжївЊГЩЗжЪЧМзЭщЃЌгыЧтЦјЯрБШЃЌЯрЭЌЬѕМўЯТЕШжЪСПЕФМзЭщШМЩеЪЭЗХГіЕФШШСПНЯЖр
B.ЪЏгЭЗжСѓПЩЛёЕУЦћгЭЁЂУКгЭЁЂЪЏРЏЕШПѓЮягЭЃЌУКНЙгЭИЩСѓПЩЛёЕУБНЁЂМзБНЕШгаЛњЮя
C.АБЛљЫсВаЛљдкЕААзжЪыФСДжаЕФХХСаЫГађГЦЮЊЕААзжЪЕФвЛМЖНсЙЙЃЌгЂЙњПЦбЇМвЩЃИёвђВтГіХЃвШЕКЫиЕФвЛМЖНсЙЙЖјЛёЕУХЕБДЖћЛЏбЇНБ
D.ЯЫЮЌЫидквЛЖЈЬѕМўЯТПЩгыХЈЯѕЫсЗЂЩњѕЅЛЏЗДгІЕУЕНЯѕЛЏЯЫЮЌЃЌЦфжаЕФЖЬЯЫЮЌГЦЮЊШЫдьУоЃЌГЄЯЫЮЌГЦЮЊШЫдьЫП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЪЧвдЧІаюЕчГиЮЊЕчдДЃЌФЃФтТШМюЙЄвЕЕчНтБЅКЭЪГбЮЫЎЕФзАжУЭМ(CЁЂDОљЮЊЪЏФЋЕчМЋ)ЁЃ
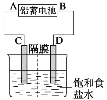
вбжЊЃКЧІаюЕчГидкЗХЕчЪБЗЂЩњЯТСаЕчМЋЗДгІЃК
ИКМЋЃКPbЃЋSO42--2e-=PbSO4 е§МЋЃКPbO2ЃЋ4HЃЋЃЋSO42-ЃЋ2e-=PbSO4ЃЋ2H2O
(1)ЧыаДГіЕчНтБЅКЭЪГбЮЫЎЕФЛЏбЇЗНГЬЪН__ЁЃ
(2)ШєдкЕчНтГижаCМЋвЛВрЕЮЗгЬЊЪдвКЃЌЕчНтвЛЖЮЪБМфКѓЮДГЪКьЩЋЃЌЫЕУїЧІаюЕчГиЕФAМЋЮЊ__МЋЁЃ
(3)гУЧІаюЕчГиЕчНт200mlХЈЖШЮЊ0.5mol/LCuSO4 ШмвКвЛЖЮЪБМфКѓ
ЂйШєСНМЋЪеМЏЕНЕФЦјЬхЬхЛ§ЯрЕШЃЌдђзЊвЦЕчзг_____molЁЃ
ЂкбєМЋЪеМЏЕНЕФЦјЬхЬхЛ§(БъзМзДПіЯТ)ЮЊ_____LЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com