
����Ŀ��������A��Ԫ���γɵĻ���������Ҫ�İ뵼�������Ӧ����㷺�����黯��(GaAs)���ش��������⣺
��1����̬Gaԭ�ӵĺ�������Ų�ʽΪ__________����̬Asԭ�Ӻ�����__________��δ�ɶԵ��ӡ�
��2����ʧȥ���ӵ�������(��λ��kJ��mol-1)����ֵ����Ϊ577��1985��2962��6192���ɴ˿���֪�ص���Ҫ���ϼ�Ϊ__________��+3����ĵ縺�Ա���__________(������������С��)��
��3���Ƚ������ص�±������۵�ͷе㣬������仯���ɼ�ԭ��__________________________��
�ص�±���� | GaCl3 | GaBr3 | GaI3 |
�۵�/�� | 77.75 | 122.3 | 211.5 |
�е�/�� | 201.2 | 279 | 346 |
GaF3���۵㳬��1000�������ܵ�ԭ����_______________________________________��
��4����ˮ�ϲ����صĽṹ��ͼ��ʾ��������ԭ�ӵ���λ��Ϊ__________���������̼ԭ�ӵ��ӻ���ʽΪ__________��

��5���黯���۵�Ϊ1238�������������ṹ��ͼ��ʾ����������Ϊa=565pm���þ��������Ϊ__________��������ܶ�Ϊ__________(��NAΪ�����ӵ���������ֵ���г���ʽ����)g��cm-3��

���𰸡� [ Ar ]3d104s24p1(��1s22s22p63s23p63d104s24p1) 3 +1(д+1��+2) �� GaCl3��GaBr3��GaI3���ۡ��е��������ߡ����Ǿ�Ϊ���Ӿ������ṹ��������Է��������������������Ӽ�������������ǿ GaF3Ϊ���Ӿ��� 4 Sp2 ԭ�Ӿ��� 
��������(1) Ga��ԭ������Ϊ31�����Ի�̬ԭ�ӵĵ����Ų�ʽΪ[ Ar ]3d104s24p1(��1s22s22p6 3s23p6 3d104s24p1)��As��ԭ������Ϊ33�����̬ԭ�ӵĵ����Ų�ʽΪ[ Ar ]3d104s24p3�����Ի�̬Asԭ�Ӻ�����3��δ�ɶԵ��ӣ�
(2)����������̬ԭ��ʧȥ��������Ҫ�����������ص�ǰ�ļ������ܿ�֪������Ҫ���ϼ�Ϊ+1��+3������As�����������Ų�Ϊ4s24p3����ȫ�����������Ga�����������Ų�Ϊ4s24p1���ر���4p1��ʧ������������ĵ縺�Ա��ش�
(3)����������ʾ���ص�±������۵�ͷе㶼���ߣ��Ұ����ȡ��塢���������ߣ�ԭ�������ǵ������ͬ���ṹ���ƣ����Ƿ��Ӿ��壬����������Է��������������Ӽ������������۷е����ߣ���GaF3���۵㳬��1000����������F�ĵ縺�Ժܴ��γɵ�GaF3�����Ӿ��壻
(4)�ɶ�ˮ�ϲ����صĽṹͼ�ɵã���ԭ�ӵ���λ��Ϊ4���������̼ԭ�����Ȼ��е�̼ԭ�ӵ��ӻ���ʽ��ͬ���γɵĶ���ƽ��ṹ������Ӧ����sp2�ӻ���
(5)���ڸþ�����۵�ߣ�������ض����ǻ���Ԫ�أ����Ըþ�����ԭ�Ӿ��壬�仯ѧʽΪGa4As4���þ���������m=![]() g�����ΪV =(565��10-10)3 cm3�������ܶ�Ϊ
g�����ΪV =(565��10-10)3 cm3�������ܶ�Ϊ![]() g/cm3��
g/cm3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ�ڻ�����������Ӧ�ù㷺��
��1����ϩ�ܺϳɺܶ���ʵ�ü�ֵ���л��

���Լ�a��_____________��
�ڷ�Ӧ��ķ�Ӧ������_____________��
��2����ԭ��Ϊ��ʼԭ�Ϻϳɾ���ϩ��·������ͼ��ʾ��

�پ���ϩ�Ľṹ��ʽ��_____________��
��д������ʽ����C4H10���л���Ľṹ��ʽ_____________��
��3����֪��CH3CHO![]() CH3COOH����CH2��CH2Ϊ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�����������д���ϳ�·�ߣ��ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
CH3COOH����CH2��CH2Ϊ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�����������д���ϳ�·�ߣ��ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������
____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������о���Ա������һ�ִ�����Ũ����ȩ��ˮ���·���һ��Ĥ��ⷨ����ȩ�ֱ�����������������Ӧ��ת��Ϊ�Ҵ������ᡣʵ������һ��Ũ�ȵ���ȩ��Na2SO4 ��ҺΪ�������Һ��ģ����ȩ��ˮ�Ĵ������̣���װ����ͼ��ʾ������˵����ȷ����

A. �������У��������ֱ�����������Ҵ��⣬����������ɫ���壬��������������O2
B. ������ӦΪCH3CHO-2e-+2H+=CH3COOH+H2O
C. �������У�������Na2SO4�����ʵ�������
D. ����CH4������ȼ�ϵ��Ϊֱ����Դ��ȼ�ϵ�ص�b ��Ӧͨ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ����ͬ��þ������������Һ��Ӧ����Ӧ��ʼʱ�ų�H2�����ǣ� ��
A.10mL 1mol/L ����B.10mL 1mol/L ����
C.10mL 3mol/L ����D.40mL 1mol/L ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ��ص�����ת�����ڣ� ��
A. ����ת��Ϊ��ѧ�� B. ��ѧ��ת��Ϊ��ѧ��
C. ��ѧ��ת��Ϊ���� D. ����ת��Ϊ��е��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ӵ�ع㷺������Яʽ��Դ�������������Ǿ�������ӵ�ؿ���������ѭ�������Ĺؼ�����֮һ��﮶��ε��һ����LiCoO2��LiFePO4��Ϊ�������ϣ���ʯī̼Ϊ�������ϣ�������LPF6��LiBF4�ȵ�̼�������(DEC)Ϊ���Һ�����ʱ��Li+��������״������ľ�����ѳ������л����Һ���л����Һ�е�Li+����븺�����õ��Ӻ���ԭ����ʽǶ�뵽ʯī���ϵľ����У���:6C+xLi++xe-=LixC6����ͼ��ʾ:

��1����ͼ��ʾ����֪�õ�ص缫�ܷ�Ӧ:LiCoO2+C![]() Li1-xCoO2+CLix�����ʱ���õ�ص������ϵķ�ӦΪ_______________________��
Li1-xCoO2+CLix�����ʱ���õ�ص������ϵķ�ӦΪ_______________________��
��2���ŵ�ʱ������������________(����������������С������������)
��3����ʵ�����У��������з����ӷϾ�����ӵ�ص�����������(��Ҫ����LiCoO2��̿�ۼ�����Al��Fe��)�����ܺ�ﮡ�
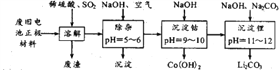
���ܽ�����У�ͨ��SO2ʱ��������Ӧ�Ļ�ѧ����ʽΪ________________________��
�ڳ��ӹ����У����ó�������Ҫ�ɷ���___________________��(д��ѧʽ)
�۳����£���֪Ksp[Co(OH)2]=1.09��10-15����������ʱpH=9.5������Һ��Co2+�Ƿ������ȫ?����ʽ����˵���� _______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
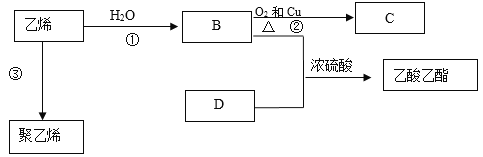
����Ŀ����ϩ�IJ����Ǻ���һ������ʯ�ͻ���ˮƽ�ı�־����ϩ������ת����ϵ��
��ش��������⣺
��1����ϩ�Ľṹ��ʽΪ______��
��2��B���������ŵ�����Ϊ____________��
��3���۵ķ�Ӧ������____________��
��4��Ũ�����������________________��
��5����Ӧ�ٵĻ�ѧ����ʽΪ________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽΪ_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ں��ݾ��������£�һ����˵����ӦA(g)��B������![]() 2C(g)�Ѵﵽƽ��״̬����
2C(g)�Ѵﵽƽ��״̬����
A. �����ڵ��ܶȲ��ٱ仯 B. C������������A�ķֽ�����֮��Ϊ2:1
C. ������ƽ��Ħ���������ٱ仯 D. �����ڵ��¶Ȳ��ٷ����仯
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ϳɰ���ҵ�Թ��ú���ᷢչ������Ҫ�����塣��ԭ��Ϊ��N2(g)+3H2(g)![]() 2NH3(g) H����92.2 kJ��mol��1 ���ݴ˻ش��������⣺
2NH3(g) H����92.2 kJ��mol��1 ���ݴ˻ش��������⣺
��1����ij�¶��£����� 10 mol N2 �� 30 mol H2�������Ϊ 10 L ���ܱ������ڣ���Ӧ�ﵽƽ��״̬ʱ����û�������а����������Ϊ 20��������¶��·�Ӧ��K=_______ (���÷�����ʾ)��
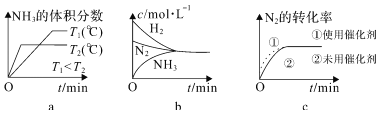
�ڶ��ںϳɰ���Ӧ���ԣ������й�ͼ��һ����ȷ����______��ѡ����ţ���
���ڼ�����Һ��ͨ����ⷨ����ʵ���� N2 ��ȡ NH3��2N2+6H2O![]() 4NH3+3O2�������ĵ缫��Ӧʽ��_______________��
4NH3+3O2�������ĵ缫��Ӧʽ��_______________��
��2�������£����� 0.1 mol��L��1 ������� 20 mL 0.1 mol��L��1 ��ˮ�У���Һ pH �������������ı仯��������ͼ��ʾ��
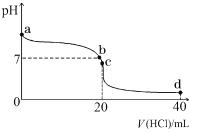
��NH3��H2O �ĵ��뷽��ʽ��_______________��
��b ����ʾ��Һ�е�������_______________��
��c ����ʾ��Һ�У�����Ũ�ȴӴ�С�Ĺ�ϵΪ_______________��
�ܳ����£����� amol/LNH3��H2O �������� bmol/L �������ϣ���ַ�Ӧ����Һ������(�����ǰ�ˮ������Ļӷ�)������¶��� NH3��H2O �ĵ��볣��Ka=___________���ú� a �� b �Ĵ���ʽ��ʾ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com