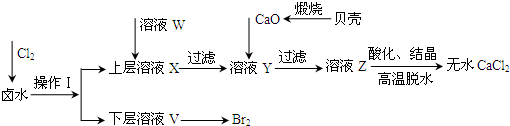
��2010?��ƽ��ģ�⣩�����Ĺ������ƣ�CaO
2���ǰ�ɫ�Ľᾧ��ĩ��������ˮ���������Ҵ������ѣ������½�Ϊ�ȶ�����һ������ˮ����ֳ���������������ʻ�ˮ��Ʒ�����䣮
��֪�����ڳ�ʪ������CaO
2�ܹ�������Ӧ��CaO
2+2H
2O��Ca��OH��
2+H
2O
2 2CaO
2+2CO
2��2CaCO
3+O
2��CaO
2��ϡ�ᷴӦ�����κ�H
2O
2��CaO
2+2H
+��Ca
2++H
2O
2��ʵ���ҿ��ø�����ȡCaO
2?8H
2O���پ���ˮ�Ƶ�CaO
2��CaO
2?8H
2O��0��ʱ�ȶ���������ʱ��������ͷֽ⣬������130��ʱ��Ϊ��ˮCaO
2��
���Ʊ��������£�

����������Ϣ���ش��������⣺
��1��������������ȡCaO
2?8H
2O�Ļ�ѧ����ʽ��
CaCl2+H2O2+2NH3+8H2O=CaO2?8H2O��+2NH4Cl����CaCl2+H2O2+2NH3?H2O+6H2O=CaO2?8H2O��+2NH4Cl
CaCl2+H2O2+2NH3+8H2O=CaO2?8H2O��+2NH4Cl����CaCl2+H2O2+2NH3?H2O+6H2O=CaO2?8H2O��+2NH4Cl
��
��2��Ϊ�˿��Ƴ����¶�Ϊ0�����ң���ʵ�����˲�ȡ�ķ�����
��ˮԡ��ȴ����Ӧ���������ڱ�ˮ�У�
��ˮԡ��ȴ����Ӧ���������ڱ�ˮ�У�
��
��3�����Ʒ��ĸ���ƷΪ
NH4Cl
NH4Cl
���ѧʽ����Ϊ����߸���Ʒ�IJ��ʣ��ᾧǰҪ����Һ��pH���������ʷ�Χ���ɼ�����Լ���
A
A
�� A������ B����ˮ
��4��Ϊ�˼��顰ˮϴ���Ƿ�ϸ�ȡ����ϴ��Һ���Թ��У��ٵμ�
ϡ�����ữ����������Һ
ϡ�����ữ����������Һ
��
��5���ⶨ��Ʒ��CaO
2�ĺ�����ʵ�鲽���ǣ�
��һ����ȷ��ȡa g��Ʒ��������ƿ�У�������������ˮ������b g KI���壬�ٵ�������2mol/L��H
2SO
4��Һ����ַ�Ӧ��
�ڶ�������������ƿ�м��뼸�ε�����Һ��
����������μ���Ũ��Ϊc mol/L��Na
2S
2O
3��Һ����Ӧ��ȫ������Na
2S
2O
3��ҺV mL������֪��I
2+2S
2O
32-��2I
-+S
4O
62-����ɫ����
�ٵ�������˵����Ӧǡ����ȫ��������
��Һ����ɫ��Ϊ��ɫ����30s���ָ�
��Һ����ɫ��Ϊ��ɫ����30s���ָ�
��
��CaO
2����������Ϊ
������ĸ��ʾ����


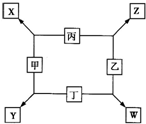 �ס��ҡ������������ɶ�����Ԫ����ɵĵ��ʣ�X��Y��Z��W��Ϊ����������£�XΪ����ɫ���壬Z��������Ϊ����ɫ��W��ʹʪ��ĺ�ɫʯ����ֽ����ɫ����ҵ�ϳ���W����ȡZ����ת����ϵ����ͼ��ʾ��
�ס��ҡ������������ɶ�����Ԫ����ɵĵ��ʣ�X��Y��Z��W��Ϊ����������£�XΪ����ɫ���壬Z��������Ϊ����ɫ��W��ʹʪ��ĺ�ɫʯ����ֽ����ɫ����ҵ�ϳ���W����ȡZ����ת����ϵ����ͼ��ʾ��