
����Ŀ����ʯ�ͺ͵��ۻ���ά��Ϊԭ�Ͼ����Ƶ�����������ת����ϵ����ͼ��
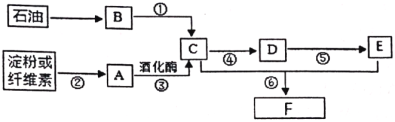
��������B�ڱ���µ��ܶ�Ϊ��1.25 g/L����ش�
��1��B�й����ŵ�������________��
��2��A�ķ���ʽ��________��
��3����Ӧ�ܵĻ�ѧ����ʽΪ________��
��4������˵����ȷ����________��
A����Ӧ�ڢݢ�����ȡ����Ӧ
B������F�Ľṹ��ʽΪCH3CH2OOCCH3
C��B��E����һ��������ֱ�Ӻϳ�F���÷���������ɫ��ѧ����
D����������������ͭ����C��D��E��������
���𰸡�̼̼˫�� C6H12O6 2CH3CH2OH + O2![]() 2CH3CHO +2H2O BCD
2CH3CHO +2H2O BCD
��������
����B�ڱ���µ��ܶ�Ϊ��1.25 g/L����Ħ������ = 1.25 g/L��22.4 L/mol = 28 g/mol����������B������ʯ���Ƶã�����Ƴ�����BΪ��ϩ�����ݷ�Ӧ�ڿ�֪����AΪ�����ǣ������ƻ�ø���û������Ҵ�����CΪ�Ҵ����Ҵ�������Ӧ�ܱ���������ȩ����DΪ��ȩ������������������Ӧ�ݣ��������ᣬ��EΪ���ᣬ��ȩ��������һ�������·���������Ӧ����������������FΪ�����������ݴ˷�������
����������������
��1��BΪ��ϩ��������ڹ���������Ϊ̼̼˫����
�ʴ�Ϊ̼̼˫����
��2��AΪ�����ǣ������ʽΪC6H12O6��
�ʴ�Ϊ��C6H12O6��
��3����Ӧ��Ϊ�Ҵ���ͭ�������ȵ������±�������ȩ�Ĺ��̣����Ӧ�Ļ�ѧ����ʽΪ��2CH3CH2OH + O2![]() 2CH3CHO +2H2O��
2CH3CHO +2H2O��
�ʴ�Ϊ��2CH3CH2OH + O2![]() 2CH3CHO +2H2O��
2CH3CHO +2H2O��
��4��A. ��Ӧ��Ϊ���۵�ˮ�ⷴӦ������ȡ����Ӧ����Ӧ��Ϊ��ȩ������Ϊ����Ĺ��̣�����������Ӧ����Ӧ��Ϊ����������������Ӧ������ȡ����Ӧ����A�����
B. ����F��Ϊ����������Ϊ�������Ҵ�ͨ�������ǻ�������õ�����Ҫ�������ӵĽṹ��ʽΪCH3CH2OOCCH3����B����ȷ��
C. BΪ��ϩ��EΪ���ᣬ����ֱ�ӻ��ϣ������ԭ��������Ϊ100%��������ɫ��ѧ�����C����ȷ��
D. ����������ͭ���Ҵ����ܣ�����ȩ�ᷢ����Ӧ����ש��ɫ�����������ᷢ������кͣ�����Һ����ɫ������Һ�����������������ͭ����C��D��E�������ʣ���D����ȷ��
��ѡB��C��D��


 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�����и������ʣ�
A ���ʯ��ʯī��B ��������ά�أ�C ���뮣�D ���������飻 E �����������
F![]() ��
�� G
G  ��
��
���л�Ϊͬλ�� _____�������ţ���ͬ������Ϊͬϵ�����____����Ϊͬ���칹�����______����ͬһ�����ʵ���________��
��2��������A�Ľṹ��ʽΪ�� ����������ȼ��Ʒ�ʿ������ܵIJ��������A��ͬ���칹���к���Ч��ԭ���������ٵ�һ�ֽṹ��ʽΪ��_____����A����ϩ����H2ͨ���ӳɷ�Ӧ�õ������ϩ�������п��ܵĽṹ��ʽΪ_____��
����������ȼ��Ʒ�ʿ������ܵIJ��������A��ͬ���칹���к���Ч��ԭ���������ٵ�һ�ֽṹ��ʽΪ��_____����A����ϩ����H2ͨ���ӳɷ�Ӧ�õ������ϩ�������п��ܵĽṹ��ʽΪ_____��
��3��������ӵļ���ʽ��ͼ��ʾ���Իش�

д��������ӵĻ�ѧʽ________��������ӵ�һ��ȡ���������Ϊ______�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��Ϊ������Ԫ�أ�������Ԫ�����ڱ��е����λ����ͼ��ʾ������B�ĵ����ڿ����к���Լռ80����
A | B | C | |||
D |
��1��д������Ԫ�ص����ƣ�C____��D___��
��2������B��ԭ�ӽṹʾ��ͼ____��C��Ԫ�����ڱ��е�λ����____��
��3��B��C����Ԫ������⻯����ȶ�����ǿ������˳����_____��д��A������⻯��ĵ���ʽ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���¹���ѧ�ҿ�������Ϊ������������6��̼ԭ���Ե�˫��������϶��ɵĻ�״�ṹ��Ϊ����֤�������йر����Ĺ۵㣬��ͬѧ�������ͼʵ�鷽����

�ٰ���ͼ��ʾ��װ��ͼ���Ӻø�������
�ڼ���װ�õ������ԣ�
����A�м��������ı���Һ��Ļ��Һ�壬�ټ����������ۣ�������Ƥ������ֹˮ��K1��K2��K3��
�ܴ�C����ƿ�ռ������������b���¶˲����ձ����ˮ�У���ѹԤ��װ��ˮ�Ľ�ͷ�ιܵĽ�ͷ���۲�ʵ������
��ش��������⡣
(1)A����������Ӧ�ķ�Ӧ����ʽΪ_____����֤�������չ۵�����ʵ��������_____��
(2)װ��B��������_________��
(3)C����ƿ������Ϊ500 mL���ռ�����ʱ�����ڿ���δ�ž�������ˮδ������ƿ��������ƿ�л�������H2������ܶ�Ϊ37.9����ôʵ�����ʱ���ɼ��������ƿ�е�ˮ�����Ϊ_______mL��(������ƽ����Է�������Ϊ29)
(4)��֪����Ľṹ��ʽΪ��![]() ���Իش� ���������������Һ��Ӧ�Ļ�ѧ����ʽ��______��
���Իش� ���������������Һ��Ӧ�Ļ�ѧ����ʽ��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ɫ��Һ�к���K+��Cl����OH����SO![]() ��SO
��SO![]() ��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��
��Ϊ������Һ��������ijЩ�����ӣ����õ��Լ��У����ᡢ���ᡢ��������Һ�����ᱵ��Һ����ˮ�ͷ�̪��Һ����������OH����ʵ�鷽��ʡ�ԣ��������������ӵĹ�������ͼ��ʾ��

��1��ͼ���Լ����������ʵĻ�ѧʽ�ֱ���
��________����________����________����__________����__________��
��2��ͼ������a��b��c��������������ӷֱ���a________��b________��c________��
��3����ɫ����A���Լ�����Ӧ�����ӷ���ʽ_________________��
��4����ɫ��ҺC���Լ�������ҪĿ����_____________________��
��5����ɫ����A�����Լ����������Լ�������ʵ���Ӱ����____________________��
��6������Eͨ���Լ���������Ӧ�����ӷ���ʽ��____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״����CH4��H2S��NH3��Ϊ���壬�ֱ�����11.2L H2S��16g CH4��1.204��1024��NH3���ӣ�������������С�Ƚ���ȷ���ǣ� ��
A. ���������������
B. �ܶȣ�����������
C. ����������������
D. ԭ������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��С��ͬѧ����CuSO4��5H2O����500mL 1 mol/L����Һ��
��1��С��ͬѧѡ�õIJ��������У��ձ�����������__________��________��
��2���������ڸ�ʵ��������Ҫ����;���ֱ���__________�� _________��
��3��С��ͬѧͨ�����㣬��������ƽ��ȡ________gCuSO4��5H2O��
��4�����ʵ���Ũ������ƫ�ߡ�ƫ�͡���Ӱ�죩
��������ƿ��ϴ����δ�����������ˮ���������Ƶ���ҺŨ�Ƚ�_________��
�ڶ���ʱ�����۾����ӣ��������Ƶ���ҺŨ�Ƚ�___________��
��ҡ�Ⱥ�������ڿ̶��ߣ��ټ�������ˮ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������(�ֳ��£�N2H4����ɫҺ��)��һ��Ӧ�ù㷺�Ļ���ԭ�ϣ����������ȼ�ϡ�
��1���������ӵĵ���ʽΪ______________��
��2������Ϊ��Ԫ�����ˮ�еĵ��뷽ʽ�백���ơ�д����ˮ��������һ�����뷴Ӧ�ķ���ʽ______________________________________________________��
��3����֪12.8 g��Һ̬����ȼ��������������ȼ�գ�������̬N2��Һ̬ˮ���ų�248.8kJ��������д����ʾҺ̬����ȼ���ȵ��Ȼ�ѧ����ʽ___________________��
��4����֪��2O2(g)+N2(g)=N2O4(l) ����H1
��N2(g)+2H2(g)=N2H4(l) ����H2
��O2(g)+2H2(g)=2H2O(g) �� ��H3
��2 N2H4(l) + N2O4(l)= 3N2(g)+ 4H2O(g) ����H4
������Ӧ��ЧӦ֮��Ĺ�ϵʽΪ��H4=_________________��������N2O4����Ϊ����ƽ�������Ҫԭ��Ϊ____________________________________________ �����ٴ�2�㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ú��˵����ȷ����( )
A.ú�ĸ���������Һ�����������仯
B.ú�к��б����ױ������ױ��ȷ�����
C.ͨ��ú�ĸ���ɻ�ñ����ױ��ȷ�����
D.ˮú����ͨ��ú�ĸ���õ�������ȼ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com