
����Ŀ����1����ĭ����������ԭ����(�����ӷ���ʽ��ʾ)_____________________��
��2������֪T��ʱ��Kw=1��10-12���ڸ��¶�ʱ����pH=9��NaOH��ҺaL��pH=2��H2SO4��ҺbL���(���Ի�Ϻ���Һ����ı仯)�������û����Һ��pH=3����a��b=____��
��ij��Ԫ�ᣨ��H2A��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�H2A=H++HA����HA- ![]() H++A2������NaHA��Һ�У�д������ԭ�ӣ����ϣ��غ�ĸ�����Ũ�ȹ�ϵ�ĵ�ʽ_____________
H++A2������NaHA��Һ�У�д������ԭ�ӣ����ϣ��غ�ĸ�����Ũ�ȹ�ϵ�ĵ�ʽ_____________
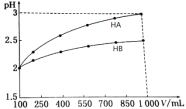
��3��pH��2������һԪ��HA��HB�������Ϊ100 mL��ϡ������pH����Һ����Ĺ�ϵ��ͼ��
����NaBˮ��Һ�Ƿ�����ԣ�ԭ��______________�������ӷ���ʽ��ʾ����
����NaOH��Һ�ζ�HB��Һ��ָʾ����ѡ��_________���ζ��յ�����Ϊ____________��
A.���� B.��̪ C.ʯ��
��4��NaHSO3��Һ�����ԣ�����Һ�У�������Ũ���ɴ�С��˳��Ϊ____________��
���𰸡�3HCO3-+Al3+=3CO2��+Al(OH)3�� 9:2 c(Na+)=c(HA-)+c(A2-) B-+H2O![]() HB+OH- B ���������һ��NaOH��Һʱ��ƿ����Һ��죬��30���ڲ��ָ�ԭɫ�� c(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)
HB+OH- B ���������һ��NaOH��Һʱ��ƿ����Һ��죬��30���ڲ��ָ�ԭɫ�� c(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)
��������
(1)��ĭ�������ԭ����NaHCO3��Һ��Al2(SO4)3��Һ��NaHCO3ˮ�ⷴӦ��HCO3-+H2O![]() H2CO3+OH-��Al2(SO4)3ˮ�ⷴӦ��Al3++3H2O
H2CO3+OH-��Al2(SO4)3ˮ�ⷴӦ��Al3++3H2O![]() Al(OH)3+3H+���ɴ˷�����
Al(OH)3+3H+���ɴ˷�����
(2) ��ǿ����ǿ���Ϻ���Һ������ԣ�ȡ�������H+����OH-�����ʵ�������Դ�С�����ݷ�ӦH++OH-=H2O��֪����n(H+)>n(OH-)�������Һ�����ԣ���n(H+)=n(OH-)�������Һ�����ԣ���n(H+)<n(OH-)�������Һ�Լ��ԡ��ڸ��ݵ��뷽��ʽ��H2A=H++HA����HA- ![]() H++A2��֪��HA-����ˮ�⣬NaHA��Һ����H2A���ӡ��ɴ˷�����
H++A2��֪��HA-����ˮ�⣬NaHA��Һ����H2A���ӡ��ɴ˷�����
(3) ��ͼ���֪HA��HB����Һ��ϡ����10����HA��Һ��pH��2���ӵ�3����c(H+)��10-2mol/L��С��10-3mol/L����H+Ũ�ȼ�С��ԭ����![]() ��˵��HA��ǿ���HB��Һϡ�ͺ�c(H+)>10-3mol/L��˵��HB��Һ�д��ڵ���ƽ���ƶ�������֪HB�����ᡣNaB����ǿ�������Σ��к͵ζ���ָʾ����ѡ��Ҫ��ѭ��ͼ�ǡ�÷�Ӧʱ��Һ�������ָʾ��������ɫ�仯��pH��Χ��һ�£��ɴ˷������
��˵��HA��ǿ���HB��Һϡ�ͺ�c(H+)>10-3mol/L��˵��HB��Һ�д��ڵ���ƽ���ƶ�������֪HB�����ᡣNaB����ǿ�������Σ��к͵ζ���ָʾ����ѡ��Ҫ��ѭ��ͼ�ǡ�÷�Ӧʱ��Һ�������ָʾ��������ɫ�仯��pH��Χ��һ�£��ɴ˷������
(4) ��ΪH2SO3������ǿ�ᣬ����NaHSO3��ˮ��Һ�м��е���ƽ�⣺HSO3-![]() H++SO32-��Ҳ��ˮ��ƽ�⣺HSO3-+H2O
H++SO32-��Ҳ��ˮ��ƽ�⣺HSO3-+H2O![]() H2SO3+OH-���ٽ�����������
H2SO3+OH-���ٽ�����������
(1)��ĭ�������ԭ����NaHCO3��Һ��Al2(SO4)3��Һ��HCO3-��Al3+������ٽ���ˮ�ⷴӦ���ܽ��е��ף����ʱ����������CO2��ĭ�Դﵽ����Ŀ�ġ��䷴Ӧԭ��Ϊ3HCO3-+Al3+=3CO2��+Al(OH)3����
(2)����T��ʱKw=1��10-12��֪�����¶���������Һc(H+)=c(OH-)=![]() =
=![]() =10-6mol/L����������Һ��pH=6����pH=9��NaOH��Һ��c(OH-)=
=10-6mol/L����������Һ��pH=6����pH=9��NaOH��Һ��c(OH-)=![]() =
=![]() =10-3mol/L��pH=2��H2SO4��Һ��c(H+)=10-2mol/L��a LNaOH��Һ��n(OH-)=10-3a mol��b LH2SO4��Һ��n(H+)=0.01b mol����Ϊ���û����Һ��pH=3<6��������Һ�����ԣ�˵��H2SO4��������c(H+)��=10-3mol/L������c(H+)��=
=10-3mol/L��pH=2��H2SO4��Һ��c(H+)=10-2mol/L��a LNaOH��Һ��n(OH-)=10-3a mol��b LH2SO4��Һ��n(H+)=0.01b mol����Ϊ���û����Һ��pH=3<6��������Һ�����ԣ�˵��H2SO4��������c(H+)��=10-3mol/L������c(H+)��=![]() =10-3mol/L�����a:b=9:2��
=10-3mol/L�����a:b=9:2��
����Ϊ��Ԫ��H2A�ĵ�һ��������ȫ���ڶ���ֻ�в��ֵ��룬��HA-����Һ��ֻ�ܲ��ֵ��������ˮ�⣺HA-![]() H++A-������NaHA��Һ�������غ��ʽΪc(Na+)=c(HA-)+c(A2-)��
H++A-������NaHA��Һ�������غ��ʽΪc(Na+)=c(HA-)+c(A2-)��
(3) ��ͼ���֪HA��HB����Һ��ϡ����10����HA��Һ��pH��2���ӵ�3����c(H+)��10-2mol/L��С��10-3mol/L����H+Ũ�ȼ�С��ԭ����![]() ��˵��HA��ǿ���HB��Һϡ�ͺ�c(H+)>10-3mol/L��˵��HB��Һ�д��ڵ���ƽ���ƶ���˵��HB�����ᡣ����ΪHB�����ᣬ�������ӦNaBΪǿ�������Σ���Һ�ʼ��ԣ�ԭ��������ӷ���ʽ��ʾ��B-+H2O
��˵��HA��ǿ���HB��Һϡ�ͺ�c(H+)>10-3mol/L��˵��HB��Һ�д��ڵ���ƽ���ƶ���˵��HB�����ᡣ����ΪHB�����ᣬ�������ӦNaBΪǿ�������Σ���Һ�ʼ��ԣ�ԭ��������ӷ���ʽ��ʾ��B-+H2O![]() HB+OH-������ΪNaOH��HBǡ���к�ʱ����NaB������Һ�Լ��ԣ�Ӧѡ���ڼ��Է�Χ�ڱ�ɫ��ָʾ��������ָʾ����ѡ���̪����ѡB���ζ��յ�����Ϊ�����������һ��NaOH��Һʱ��ƿ����Һ��죬��30���ڲ��ָ�ԭɫ��
HB+OH-������ΪNaOH��HBǡ���к�ʱ����NaB������Һ�Լ��ԣ�Ӧѡ���ڼ��Է�Χ�ڱ�ɫ��ָʾ��������ָʾ����ѡ���̪����ѡB���ζ��յ�����Ϊ�����������һ��NaOH��Һʱ��ƿ����Һ��죬��30���ڲ��ָ�ԭɫ��
(4)��ΪH2SO3������ǿ�ᣬ����NaHSO3��ˮ��Һ�м��е���ƽ�⣺HSO3-![]() H++SO32-��Ҳ��ˮ��ƽ�⣺HSO3-+H2O
H++SO32-��Ҳ��ˮ��ƽ�⣺HSO3-+H2O![]() H2SO3+OH-������ΪNaHSO3��Һ�����ԣ�����HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ�һ�����˵����̶Ⱥ�ˮ��̶ȶ����������NaHSO3��Һ������Ũ���ɴ�С��˳��Ϊc(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)��
H2SO3+OH-������ΪNaHSO3��Һ�����ԣ�����HSO3-�ĵ���̶ȴ�����ˮ��̶ȣ�һ�����˵����̶Ⱥ�ˮ��̶ȶ����������NaHSO3��Һ������Ũ���ɴ�С��˳��Ϊc(Na+)>c(HSO3-)>c(H+)>c(SO32-)>c(OH-)��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I�����ܵĴ洢������Ӧ�õ���Ҫƿ������λ�⻯��������廯������Ŀǰ�����õ���Ҫ������ϡ�
��1��Ti��BH4��2��һ�ֹ���Ԫ�����⻯�ﴢ����ϡ��ڻ�̬Ti2+�У�����ռ�ݵ�����ܲ����Ϊ_______�����ܲ���е�ԭ�ӹ����Ϊ_____��
��2��Һ���Ǹ������ʣ������ܵ��������壬����![]() ʵ�ִ�������⡣����˵����ȷ����________��
ʵ�ִ�������⡣����˵����ȷ����________��
a��NH3�����е�ԭ�ӵĹ���ӻ���ʽΪsp2�ӻ�
b��NH4+��PH4+��CH4��BH4-��ClO4-��Ϊ�ȵ�����
c����ͬѹǿʱ��NH3�ķе��PH3�ķе��
d��[Cu��NH3��4]2+�����У�Nԭ������λԭ��
��3����֪NF3��NH3�Ŀռ乹����ͬ����NF3������Cu2+�γ������ӣ���ԭ����________��
II���Ȼ����������еij��õ�ζƷ��Ҳ�ǽṹ��ѧ���о����Ӿ���ʱ���õĴ�����侧���ṹ��ͼ��ʾ��
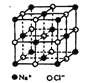
��1�����Ȼ��ƾ�����Na+���������ڵ�Cl-֮��ľ���Ϊr�����Na+������ν��ڵ�C1-����Ϊ______����Na+������ν��ڵ�Cl��֮��ľ���Ϊ_____��
��2����֪���Ȼ��ƾ�����Na+�İ뾶Ϊ��a pm��Cl-�İ뾶Ϊb pm�������ھ�
�����ǽ��ܽӴ��ģ������Ȼ��ƾ��������ӵĿռ�������Ϊ_____�����ú�a��b��ʽ��Ԭʾ��
��3�����ײ��ϵı���ԭ��ռ��ԭ�����ı����ܴ��������������������ʵ�ԭ����ij�Ȼ��ƿ�����״Ϊ�����壬�߳�Ϊ�Ȼ��ƾ�����10��������Ȼ��ƿ����б���ԭ��ռ��ԭ�����İٷֱ�Ϊ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��X��Y��Z�����ڱ���λ�ù�ϵ��ͼ��ʾ��
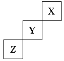
��1��XԪ�صĵ��ʷ���ʽ��___����X��������������������ȣ�X���ʵ�Ħ��������___��
��2��Y���ʵĵ���ʽ��___��
Y������ˮ��Ӧ�Ļ�ѧ����ʽ��___��
Z�����γɵĻ�����ĵ���ʽ�ǣ�___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���Ľṹ��ʽΪ CH2��CH��C��C![]() �������й��伸�νṹ��˵����ȷ���ǣ� ��
�������й��伸�νṹ��˵����ȷ���ǣ� ��
A. ����̼ԭ�Ӳ�������ͬһƽ���� B. ��4��̼ԭ����ͬһֱ����
C. ��5��̼ԭ����ͬһֱ���� D. ��6��̼ԭ����ͬһֱ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����״���£�1.92 gij��������Ϊ672 mL������������Է�������Ϊ________��
��2����25 �桢101 kPa�������£�ͬ������CH4��A��������֮����15��8����A��Ħ������Ϊ____________��
��3��������ͬ�ݻ����ܱ�����X��Y����25 ���£�X�г���a g A���壬Y�г���a g CH4���壬X��Y�ڵ�ѹǿ֮����4��11����A��Ħ������Ϊ____________��
��4����ͬ�����£������Ϊa��b��������Ϊa��b��H2��O2�Ļ�����壬��ƽ��Ħ�������ֱ���______________��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������26����ʼ���������¶��壬�����ӵ�������NA������ȷֵ6.02214076��1023������˵����ȷ����
A. 18 gT2O�к��е�������Ϊ12NA
B. ��23.5gAgI��ˮ�ƳɵĽ����н���������ĿΪ0.1NA
C. ��״���£�2.24LCl2ȫ������ˮ������Һ�е�Cl����ĿΪ0.1NA
D. 1molij����CnH2n+2��n��1�������к��еĹ��ۼ���Ϊ(3n+1)NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���绯ѧ�����ǵ�����������ռ��Խ��Խ��Ҫ�ĵ�λ��
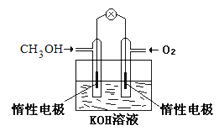
��1����ȼ�ϵ����һ����ɫ��������Ч�Ļ�ѧ��Դ����ͼΪ�״�ȼ�ϵ�أ�����ӦʽΪ____��
���ü״�ȼ�ϵ�ص��300mL����ʳ��ˮ������Ļ�ѧ����ʽΪ_________�����һ��ʱ�����Һ��pHֵΪ13ʱ������O2������Ϊ______g����������Һ����仯����������ģ�
���ü״�ȼ�ϵ�ص��2L��1mol/L����ͭ��Һ��һ��ʱ����������ռ�����״���µ�����89.6L�����·�й�ת��_______mol���ӡ�
��2�����õ绯ѧԭ������NO2��O2������KNO3�Ƴ�ȼ�ϵ�أ�ģ�ҵ��ⷨ��������Cr2O72-��ˮ������ͼ��ʾ������������Һ������Ӧ��Cr2O72-+6Fe2++14H+=2Cr3++6Fe3++7H2O��
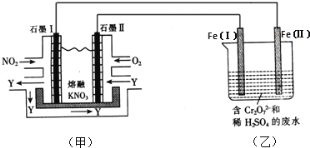
�ټ׳ع���ʱ��NO2ת�����ɫ������Y��Y��N2O5����ѭ��ʹ�ã���ʯī�������ĵ缫��ӦʽΪ__��
������ȫ��ԭΪCr3+���ҳع�ҵ��ˮ�еμ�NaOH��Һ���ɽ�����Cr��OH��3��������ʽ��ȥ����֪Cr��OH��3���������ܽ�ƽ�⣺Cr��OH��3��s��![]() Cr3+��aq��+3OH-��aq����������Cr��OH��3���ܶȻ�Ksp=c��Cr3+��c3��OH-��=1.0��10-32��Ҫʹc��Cr3+������10-5molL-1����Һ��pHӦ����________��
Cr3+��aq��+3OH-��aq����������Cr��OH��3���ܶȻ�Ksp=c��Cr3+��c3��OH-��=1.0��10-32��Ҫʹc��Cr3+������10-5molL-1����Һ��pHӦ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������в�ͬ�̶ȵĶ��ԣ����ù��ɵ�ط�������ʵ����Ч��������������ŷţ����ỷ����Ⱦ�����ܳ�����û�ѧ�ܣ�������Ӧ 6NO2+ 8NH3= 7N2+12H2O��װ����ͼ��ʾ�����й��ڸõ�ص�˵����ȷ����

A. Ϊʹ��س����ŵ磬���ӽ���Ĥ��ѡ�������ӽ���Ĥ
B. ���Ӵ��Ҳ�缫�������غ��������缫
C. �缫A����ӦʽΪ2NH3 - 6e-=N2 +6H+
D. ����4.48LNO2������ʱ��ת�Ƶ������ʵ���Ϊ 0.8mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͭ����һ����Ũ���ᣬ����NO2��N2O4��NO�Ļ�����壬��Щ��������1.12 L O2(��״��)��Ϻ�ͨ��ˮ�У����屻ˮ��ȫ���ա�����ԭ������Һ�м���5 mol��L��1 H2SO4��Һ100 mL��������ܽ��Cu������Ϊ(����)
A. 6.4 g B. 9.6 g C. 19.2 g D. 24 g
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com