
ijѧϰС����ģ���ij������Һ�л��ձ�ͪ���Ҵ��������ʵ�顣�ƶ��������������̡�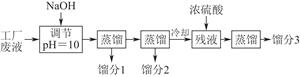
��֪�÷�Һ����Ҫ�����Ҵ������л����б�ͪ������������������Ҹ��ֳɷֵķе����±���
| ���� | ��ͪ | �������� | �Ҵ� | ���� |
| �е㣨�棩 | 56.2 | 77.06 | 78 | 117.9 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д�������ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s)  2Ca2����2K����Mg2����4
2Ca2����2K����Mg2����4 ��2H2O
��2H2O
Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
(1)������Ҫ�ɷ���________��________�Լ�δ����±ʯ��
(2)�û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��_________________________________________________��
(3)�����ӡ������У��ȼ���________��Һ��������Ȳ������ˣ��ټ���________��Һ����ҺpH�����ԡ�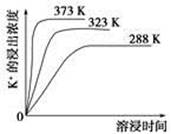
(4)��ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ����ͼ�ɵã������¶����ߣ�
��________________________________________________________��
��________________________________________________________��
(5)�����Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)�� ?
? CaCO3(s)��
CaCO3(s)��
��֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��
Ksp(CaSO4)��4.90��10��5������¶��¸÷�Ӧ��ƽ�ⳣ��K(������������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д�������ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s) 2Ca2����2K����Mg2����4SO42����2H2O
2Ca2����2K����Mg2����4SO42����2H2O
Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�
(1)������Ҫ�ɷ���________��________�Լ�δ����±ʯ��
(2)�û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��
_____________________________________________________________��
(3)�����ӡ������У��ȼ���________��Һ��������Ȳ������ˣ��ټ���________��Һ����ҺpH�����ԡ�
(4)��ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ����ͼ�ɵã������¶����ߣ�
��_______________________________________________________��
��_______________________________________________________��
(5)�����Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)��CO32�� CaCO3(s)��SO
CaCO3(s)��SO
��֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��
Ksp(CaSO4)��4.90��10��5������¶��¸÷�Ӧ��ƽ�ⳣ��K(������������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ȼ���ͭ(CuCl)�ǰ�ɫ��ĩ������ˮ���������Ҵ����ڿ����лᱻѸ����������ɫ��ʽ�Ρ������Ե�Ʒ�Һ(��Ҫ��Cu2����Fe3��)���Ʊ��Ȼ���ͭ�Ĺ�������ͼ���£�
�������Ӻ�������ҺpH��CuCl��������ҺpH�Ĺ�ϵͼ��ͼ��
����֪����������Ũ��Ϊ1 mol��L��1ʱ��Fe(OH)3��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ1.4��3.0��Cu(OH)2��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.2��6.7����ش��������⣺
(1)���ʱ������Ӧ�����ӷ���ʽ��________������CuCl����ʱ�����pH��________���ҡ�
(2)���ۡ��Ȼ��ơ�����ͭ����Һ�з�Ӧ����CuCl�����ӷ�Ӧ����ʽΪ____________________________��
(3)������CuCl����Ҫ��������ˮ�Ҵ�ϴ�ӣ�����ո��������70�����2 h����ȴ�ܷ��װ��70����ո���ܷ��װ��Ŀ����____________________________________________��
(4)��Ʒ�˳�ʱ������Һ����Ҫ�ֳ���________���������Һ�л�ȡFeSO4��7H2O���壬����Ҫ֪������__________________��
(5)�������ۻ�����������Ҳ�ɵõ��Ȼ���ͭ����д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��Ϊ���CuCl�IJ��ʣ����ڸ÷�Ӧ��ϵ�м���ϡ����Һ������pH��3.5����������Ŀ����__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ˮ������ҵ��ɰ�ǡ���֬��Ư����ɱ�������У���������(NaClO2)��������Ҫ�����á���ͼ�������������ƵĹ�������ͼ��
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2��3H2O��
�ڳ����£�Ksp(FeS)=6��3��10-18��Ksp(CuS)=6��3��10-28��Ksp(PbS)=2��4 ��10-28
��1����ӦI�з�����Ӧ�����ӷ���ʽΪ ��
��2������Һ�еõ�NaClO2��3H2O������������������ (��д���)��
a������ b������Ũ�� c������ d����ȴ�ᾧ e������
��3��ӡȾ��ҵ������������(NaClO2)Ư��֯�Ư��֯��ʱ���������õ���HClO2���±���25��ʱHClO2�����ֳ�������ĵ���ƽ�ⳣ����
| ���� | HClO2 | HF | H2CO3 | H2S |
| Ka��mol��L-1 | 1��10-2 | 6.3��10-4 | K1=4.30��10-7 K2=5.60��10-11 | K1=9.1��10-8 K2=l.1��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�Ǿ����Դ���⣬�ں�ˮ�������ۺ����÷��棬�����λ��ȫ��ǰ�С��Ӻ�ˮ����ȡʳ�κ���Ĺ������£�
��1�����оٺ�ˮ���������ַ�����________��________��
��2����NaCl��Һ���е�⣬�ڵ����п�ֱ�ӵõ��IJ�Ʒ��H2��________��________��H2��________��
��3����������ѻ��Br2����������ֽ�Br2��ԭΪBr������Ŀ��Ϊ____________________________________________________________________��
��4���������SO2ˮ��Һ����Br2�������ʿɴ�95%���йط�Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
�ɴ˷�Ӧ��֪�������������⣬�ڹ�ҵ������Ӧ�������Ҫ������_________________________________________________________________��
��5��ij��ѧ�о���ѧϰС��Ϊ�˽�ӹ�ҵ�����ᴿ��ķ������������й����ϣ�Br2�ķе�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԡ����Dzι��������̺���������װ�ü�ͼ��
�������������ۣ�
��ͼ������B������________��
������ʵ��װ�����������Ӿ��������������ܣ���ԭ����____________________________________________________________��
��ʵ��װ�����������ã�Ҫ�ﵽ�ᴿ���Ŀ�ģ���������ο��ƹؼ�����________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������dz��õ�����������ҵ�������̿���Ҫ�ɷ���MnO2��Ϊԭ���Ʊ�������ؾ��塣��ͼ��ʵ�����Ʊ��IJ������̣�
������Ӧ�ڵĻ�ѧ����ʽ��
��֪��
��1���������̿�KClO3��KOH����ʱ�������ô�������ѡ����������������_______��
��Ӧ�ٵĻ�ѧ����ʽΪ______��
��2������Һ�еõ�KMnO4�����ʵ�����������________��ѡ����ĸ���ţ���ͬ����
A������ B������ C������ D������ E����ȴ�ᾧ
��3���Ʊ���������Ҫ�õ�������CO2���塣��ȡ��CO2�����ѡ�������Լ���_________��
A��ʯ��ʯ B��Ũ���� C��ϡ���� D������
��4��ʵ��ʱ����CO2����������KHCO3�����µõ���KMnO4��Ʒ�Ĵ��Ƚ��͡�ԭ����______ ��
��5������CO2��ͨ�������ѿ��ƣ���˶�����ʵ�鷽�������˸Ľ�������ʵ����ͨCO2��Ϊ���������ᡣ�������Ϸ�����ѡ����������________ ���õ��IJ�Ʒ���ȸ��ߡ�
A������ B��Ũ���� C��ϡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CaCl2�����ڶ�����·��ѩ��������ҵ�ķ����ȣ�ʵ���ҳ��������������ҵ�ϳ��ô���ʯ����������Al3+��Fe2+��Fe3+�����ʣ����Ʊ�����ͼΪʵ����ģ���乤�����̣�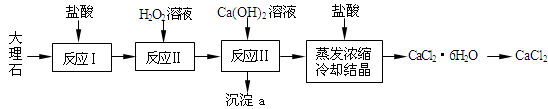
��֪�������£���Һ�е�Fe3+��Al3+��Fe2+������������ʽ��ȫ������pH�ֱ�Ϊ��3.7��4��9.7��
��1����Ӧ���У��轫����ʯ���顢���裬ͬʱ�ʵ����ȣ���Ŀ���ǣ� ��д����Ӧ������Ҫ��Ӧ�����ӷ���ʽ�� ��
��2������ʹ�������Ũ��Ϊ10%������37%��Ũ����������500mL�Ĵ���������IJ��������У�����������Ͳ���ձ�����ͷ�ιܡ� ��
��3����Ӧ���е����ӷ���ʽ�� ��
��4����Ӧ���б�����Ƽ���Ca(OH)2������ʹ��Һ��pHԼΪ8.0����ʱ����a�ijɷ�Ϊ�� ����pH��������ܷ�������Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ѡ������ʵ�鷽���������ʣ������뷽����������ں����ϡ�
| A����ȡ��Һ | B������ | C���ᾧ | D����Һ E������ F������ G������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com