
��1��������ijǿ����ҺpH��a��ǿ����ҺpH��b����֪a��b��12�������Һ���pH��7��������Һ���V(��)�ͼ���Һ���V(��)�ı�ֵ��ϵΪ ��
��2������Ũ�Ⱦ�Ϊ0.1 mol/L��������Һ���������������ڴ�����������ƣ������ᣬ������Һ����ˮ�������OH��Ũ���ɴ�С��˳���ǣ�����ţ� ��
��3����֪100�� KW��10��12���������¶���pH��8��Ba(OH)2��Һ��pH��5��ϡ�����ϣ�������100��ĺ��£���ʹ�����ҺpH��7����Ba(OH)2��Һ����������֮��Ϊ ��
��1�� V(��):V(��)=1:100 ��2�� ��>�ۣ���>�� ��3��2:9
���������������1��������ijǿ����ҺpH��a������Һ��������Ũ����10��amol/L��ǿ����ҺpH��b������Һ��OH��Ũ����10b��14mol/L����֪�����Һ���pH��7����V(��)��10��amol/L��V(��)��10b��14mol/L������Ϊa��b��12������V(��):V(��)=10a��b��14��10��2��1:100��
��2�����������Ƕ�Ԫǿ�����Һ��OH��Ũ����0.2mol/L�����������ᣬ���ڵ���ƽ�⣬����Һ��������Ũ����С��0.1mol/L������������һԪǿ�����Һ��OH��Ũ����0.1mol/L��������һԪǿ�ᣬ����Һ��������Ũ����0.1mol/L��һԪ�������������ӻ��������OH������ˮ�ĵ��룬�������ӻ�OH��Ũ��Խ�����Ƴ̶�Խ�����������Һ����ˮ�������OH��Ũ���ɴ�С��˳���Ǣ�>�ۣ���>�١�
��3����֪100�� KW��10��12������¶���pH��8��Ba(OH)2��Һ��OH��Ũ����10��4mol/L��pH��5��ϡ������Һ��������Ũ����10��5mol/L�������Ϻ���Һ��pH��7����˵�����ǹ����ģ������� ��10��5mol/L�����V(��):V(��)=2:9��
��10��5mol/L�����V(��):V(��)=2:9��
���㣺����ˮ�ĵ��롢��ҺpH�ļ���


 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������±ʯ(K2SO4��MgSO4��2CaSO4��2H2O)���ڡ�������ˮ�д�������ƽ�⣺
K2SO4��MgSO4��2CaSO4��2H2O(s) 2Ca2����2K����Mg2����4SO42����2H2O
2Ca2����2K����Mg2����4SO42����2H2O
Ϊ�ܳ�����ü���Դ���ñ���Ca(OH)2��Һ�ܽ���±ʯ�Ʊ�����أ������������£�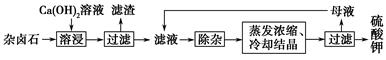
(1)������Ҫ�ɷ���________��________�Լ�δ����±ʯ��
(2)�û�ѧƽ���ƶ�ԭ������Ca(OH)2��Һ���ܽ���±ʯ����K����ԭ��_________________________________________________��
(3)�����ӡ������У��ȼ���________��Һ��������Ȳ������ˣ��ټ���________��Һ����ҺpH�����ԡ�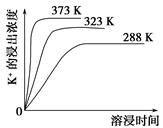
(4)��ͬ�¶��£�K���Ľ���Ũ�����ܽ�ʱ��Ĺ�ϵ��ͼ����ͼ�ɵã������¶����ߣ�
��________________________________________________________��
��________________________________________________________��
(5)�����Կ�����̼����Ϊ�ܽ��������ܽ������лᷢ����CaSO4(s)��CO32�� CaCO3(s)��SO42��
CaCO3(s)��SO42��
��֪298 Kʱ��Ksp(CaCO3)��2.80��10��9��
Ksp(CaSO4)��4.90��10��5������¶��¸÷�Ӧ��ƽ�ⳣ��K(������������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������£�����������Һ��
��0��1mol/L NH4Cl
��0��1mol/L CH3COONH4
��0��1mol/L NH4HSO4
��0��1mol/L NH3��H2O��0��1mol/L NH4Cl�Ļ��Һ
�����Ҫ����д���пհף�
��1����Һ�ٳ����ԣ���ԭ����_______________________________(�����ӷ���ʽ��ʾ)
��2���Ƚ���Һ�ڡ�����c(NH4+)�Ĵ�С��ϵ�Ǣ� ��(�������=������)��
��3������Һ���У� ���ӵ�Ũ��Ϊ0��1mol/L��NH3��H2O�� ���ӵ�Ũ��֮��Ϊ0��2 mol/L��
��4�������£������Һ�ڵ�pH=7�� CH3COO-��NH4+Ũ�ȵĴ�С��ϵ�ǣ�
c(CH3COO-) c(NH4+)(�������=������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ش��������⣺
��1�����ֱ�pH=2������ʹ���ϡ��100������ϡ�ͺ���Һ��pH������ ���ᣨ���������������������
��2����100mL 0.1mol?L-1��CH3COOH��Һ��50mL 0.2mol?L-1��NaOH��Һ��ϣ�������Һ�� �ԣ�ԭ���� �������ӷ���ʽ��ʾ����
��3��0.1mol��mol-1�İ�ˮ��Һ�д��ڵ���ƽ��NH3+H2O  NH3��H2O
NH3��H2O NH4++OH�����ڴ�ƽ����ϵ�иı����������±���������ɱ��пո�
NH4++OH�����ڴ�ƽ����ϵ�иı����������±���������ɱ��пո�
| | �����ı仯 | ͨ���� | ��ˮ | ��NH4Cl(s) |
| �� | ����ƽ���ƶ��ķ��� | | | |
| �� | c(OH��)�ı仯 | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����к͵ζ�������ʵ�������Ź㷺��Ӧ�á�����ʱ����0.250 mol/LNaOH��Һ�ζ�25.0 mL��һԪ��HR��Һʱ����Һ��pH�仯�����ͼ��ʾ������a���ʾ��������ǡ����ȫ��Ӧ����ش��������⣺
��1����һԪ��HR��Һ�����ʵ���Ũ��Ϊ_______________��
��2��ͼ��x_____7���>������<����=��������ԭ����___________________�������ӷ���ʽ��ʾ����
��3���ζ���a��ʱ����Һ��c(OH��)--c(HR)="____mol/L" (�ú�x�Ĵ���ʽ��ʾ)��
��4������ʱ��HR�ĵ��볣��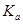 =____________mol/L��
=____________mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C�� D�� E������Һ�ֱ���NaOH��NH3·H2O��CH3COOH ��HCl��NH4HSO4�е�һ�֡������½�������ʵ�飺
�ٽ�1 L pH=3��A��Һ�ֱ���0��001mol·L��1 xL B��Һ��0��001mol·L��1 yL D��Һ��ַ�Ӧ�����ԣ�x��y��С��ϵΪ�� y��x��
��Ũ�Ⱦ�Ϊ0��1mol·L��1A��E��Һ��pH��A��E��
��Ũ�Ⱦ�Ϊ0��1mol·L��1C��D��Һ�������ϣ���Һ�����ԡ�
�ش��������⣺
��1��D�� ��Һ���ж������� ��
��2����ˮϡ��0��1 mol·L��1Bʱ����Һ������ˮ�������Ӷ���С���� ����д��ţ�
��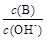 ��
�� 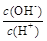 �� c��H+����c��OH-���ij˻� �� OH�������ʵ���
�� c��H+����c��OH-���ij˻� �� OH�������ʵ���
��3��OH��Ũ����ͬ�ĵ������������ҺA��E���ֱ���п�۷�Ӧ����������һ����Һ�д���п�ۣ��ҷų�������������ͬ��������˵����ȷ����________����д��ţ�
�ٷ�Ӧ����Ҫ��ʱ��E>A
�ڿ�ʼ��Ӧʱ������A>E
�۲μӷ�Ӧ��п�����ʵ���A=E
�ܷ�Ӧ���̵�ƽ������ E>A
��A��Һ����п��ʣ��
��E��Һ����п��ʣ��
��4����������������ʵ���Ũ��B��C��Ϻ���Һ�������¶ȣ����ʲ���ֽ⣩��ҺpH���¶ȱ仯��ͼ�е�_________���ߣ���д��ţ� �������£���0��01mol·L��1 C��Һ�еμ�0��01mol·L��1 D��Һ�����ԣ��õ�����Һ���������ӵ����ʵ� ��Ũ���ɴ�С��˳��Ϊ ��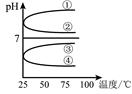
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ϊ��֤��������������ʣ��ס��ҡ����������˷ֱ�ѡ�������Լ�����ʵ�飺
0.10mol/L������Һ��pH=3�����ᡢpH=3�Ĵ��ᡢNaAC���塢NaCL���塢pH��ֽ������ˮ��
��1������pH��ֽ���0.10mol/L�Ĵ�����ҺpH=4�����϶�������������ʣ�����Ϊ��һ������ȷ�𣿣����ȷ������ȷ����
��2����ȡ��10ml0.10mol/L�Ĵ�����Һ����pH��ֽ�����pH=a��Ȼ��������ϡ�͵�1000mL������pH��ֽ�ⶨ��pH=b��Ҫȷ��������������ʣ���a��bӦ������Ĺ�ϵ�� ���á���ʽ������ʽ����ʾ��
��3������pH=3��������ᣬ��ȡ10ml��������ˮϡ�͵�ԭ����100����Ȼ����pH��ֽ�ⶨ����Һ��pH������ı仯С�����϶�������������ʣ�����Ϊ��һ������ȷ��
�����ȷ������ȷ����
��4������pH=3��������ᣬ�ֱ������Ӧ�����ι��壬�����PH�仯�����϶�������������ʣ�����Ϊ��һ������ȷ�𣿣����ȷ������ȷ���� ��
��5����ȡpH=3������ʹ��ᣬ�ֱ�ϡ�͵�ԭ����100����Ȼ�������ȫһ����п��������ų�H2�����ʿ죬���϶�������������ʣ�����Ϊ��һ������ȷ�𣿣����ȷ������ȷ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����±��ش���������(��Ϊ�����µ�����):
| �� | ���볣��(Ka) | �� | ���볣��(Ka) |
| CH3COOH | 1.8��10-5 | H2CO3 | K1=4.4��10-7 K2=4.7��10-11 |
| HClO | 3��10-8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��22�֣��о�̼���仯��������ʺ���;����ѧ��ѧ������֮һ��
I��ij��ȤС����ʵ�������Ʊ�̼������Һ���������£�����һ��ȡ25 mLһ��Ũ�ȵ�NaOH��Һ��ͨ��CO2�������������������������в���һ������Һ������������ȡ25 mL��ͬŨ�ȵ�NaOH��Һ�벽���������Һ��ϣ�����̼������Һ��
��1����ɲ���һ��ѡ�Ļ�ѧ�Լ��У�ϡ���ᡢNaOH��Һ������ʯ������̼������Һ��ϡ���ᡢ����̼��������Һ�ȣ���Ҫ��װ��������ʾ��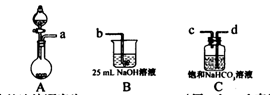
�ٸ�װ����ȷ������˳��Ϊ ����a��b��c��d��ʾ����
�ڼ������װ�������Եķ����� ��
��װ��A�г���ҩƷΪ ��װ��C�л�ѧҩƷ�������� ��
��2��д���������з�����Ӧ�����ӷ���ʽ ��
��3����ͬѧ������������û�б�Ҫ����������Լ��Ŀ��� ��
��ʵ�����ô�������ᴦ��ij������ʯ����֪����ʯ�к���MgO��SiO2��CaO��Fe2O3��Al2O3�������ģ���Ʊ�����þ���������£�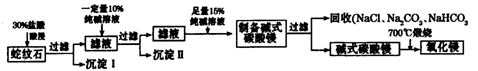
��1��������Ҫ�IJ��������� ��
��2��������ijɷ�Ϊ ���������ӷ���ʽ��ʾ���ɳ����Ĺ��� ��д��һ�����ɣ���
��3��������Һ����������Ũ�ȵĴ�С��ϵΪ ��
��4����֪l0%�Ĵ�����Һ�ܶ�Ϊ1��06g��cm3���������ʵ���Ũ��Ϊ____ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com