
����Ŀ��ij�²�ϸ�����ڵ��ⶾ�أ���ɫ��ϸ��״�ᾧ����С��������к�ǿ�Ķ��ԣ����������ѡ�Ż�¡���Ѫ�����εȣ��������������ⶾ��Ϊ��״�ģ��ṹʽ��ͼ��ʾ�����ͼ�����ش�

��1���û������к�������İ���_____________�����Ȼ�________________����
��2���û���������_____________����������ɵģ�������Щ�������������������ṹ�е�_____________��
��3����ɸû�����İ�������___________�֣�������_____________���������R����ͬ�����R����_______________��
��4���û������Ϊ��״__________�Ļ��������___________���ļ���
��5����д���߿��ڽṹ�����ƣ�A._________________��B.___________________��
��6���û��������8����ԭ�ӣ�����_____________��λ���ļ��ϣ�____________��λ��R���ϡ�
��7�����ⶾ�ػ������γɹ�����ʧȥ��______________��ˮ���ӡ�
���𰸡�(1)0 0 (2)7 R�� (3)5 3 ��CH3 (4)�� 7(5)�ļ� R�� (6)7 1 (7)7
��������
��1������İ����ṹΪ��NH2��������Ȼ��ṹΪ��COOH��
��2���û�����7���ļ�����CO��NH���������Ʋ���7����������ɣ������ͬ��R���йء�
��3������R�����Ʋⰱ������5�֣�������3����������R����ͬ�����ǣ�CH3��
��4���û�������7����������ˮ�����γɣ���Ϊ���ģ�����7���ļ���
��5��A���ļ���B��R����
��6��ÿ���ļ�����1����ԭ�ӣ�����7���ļ�����7����ԭ�ӣ�1��λ��R���ϡ�
��7����״�����γɹ����й���ȥ7��ˮ���ӡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������9.11���ֲ�Ϯ���¼��У��ٻ��Ľ����������ʯ�ޣ�������ʯ����ά���ΰ���ʯ���ǹ����ο��ij��ʯ�Ļ�ѧʽΪ��CaMgxAlySi3O12���û�ѧʽ��x��y��ֵ�ֱ���( )
A.2��2B.2��3C.3��2D.4��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�������ӵĻ�ԭ����ǿ��˳��ΪI��>Fe2��>Br��������200 mL�����Һ�к�FeI2��FeBr2��0.10 mol����������ε�����ˮ(�ٶ�Cl2����ֻ���������ӷ�Ӧ��������������Ӧ)
��1������ˮ����0.15 mol Cl2����ԭ����������Һ�к��е���������Ҫ��________��ʣ��Fe2�������ʵ���Ϊ________��
��2����ԭ��Һ��Br����һ�뱻������������Cl2�����ʵ���Ϊ________��������������ҺΪ400 mL��������Ҫ�����Ӽ������ʵ���Ũ��Ϊ________��
��3��ͨ����������Ӧ�ķ��������ж�Cl2��I2��Fe3����Br2����������������������ǿ������˳����_________________________________________________��
��4��������Ӧ��ԭ��Һ����������ȫ�����������ٵ���������ˮ����I2ȫ����Cl2������HIO3(ǿ��)����д���˷�Ӧ�����ӷ���ʽ��_________________���������з�Ӧ������Cl2________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ͬ��ѧԪ������ɵĻ��������˵������ȷ����

A. ��ͼ�Т�Ϊij�ֶ����ĵ��壬���������ǰ�����
B. ���ڴ�����Ƥ�º�����������Χ�Ȳ�λ�������֬��
C. ��һ���Ǻ��������ɺ���ĵ��������
D. �����Dz��빹��ֲ��ϸ���ڵ�һ�ֶ��ǣ�������������ά��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ú̿ȼ�ղ�����SO2��CO��NO2����������صĴ�����Ⱦ���⡣
(1) CaO��������SO2�ŷ��������á�
��֪����SO2(g)+ CaO(s)=CaSO3(s) ��H=-402 kJ��mol-1
��2CaSO3(s)+O2(g)=2CaSO4(s) ��H=-234 kJ��mol-1
��CaCO3(s)=CO2(g) +CaO(s) ��H = +178 kJ��mol-1
��Ӧ2SO2(g)+O2(g)+2CaO(s)= 2CaSO4(s) ��H =________ kJ��mol-1
��ȼú�м���CaCO3Ҳ���������������̶�2molSO2��Ӧ����ú����ͬ��������ȼ��ʱ���ͷų������������______ kJ��
(2)����CO��H2�ڴ��������ºϳɼ״����Ǽ�����Ⱦ��һ���¾ٴ�����Ӧԭ��ΪCO(g)+2H2(g) ![]() CH3OH(g)��H���������ͬ�����������ܱ������зֱ����1molCO��2mol H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ�ס�
CH3OH(g)��H���������ͬ�����������ܱ������зֱ����1molCO��2mol H2�����ƽ��������CH3OH����������ڲ�ͬѹǿ�����¶ȵı仯��ͼ�ס�
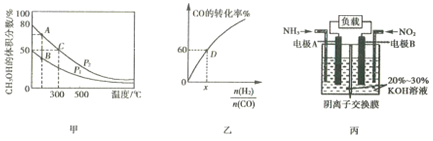
�������ϳɼ״��ķ�Ӧ��______(��������������������)��Ӧ���жϵ�������_____��
��ͼ����A��B��C�����з�Ӧ����������______(��д��A������ B�� ���� C��)��
����300��ʱ����C��ƽ����ϵ���ٳ���0.25molCO��0.5molH2��0.25molCH3OH����ƽ��______(����������Ӧ�������������淴Ӧ��������������)�ƶ���
(3)һ���¶��£�CO��ת��������ʼͶ�ϱ�![]() �ı仯��ϵ��ͼ����ʾ�����D��������ת����Ϊ40%����x=______��
�ı仯��ϵ��ͼ����ʾ�����D��������ת����Ϊ40%����x=______��
(4)����ԭ���ԭ���ɽ�NO2��NH3ת��Ϊ����Ⱦ���ʣ���װ��ԭ��ͼ��ͼ����ʾ������ӦʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����÷ϱ���(��Ҫ�ɷ�ΪBaS2O3��������SiO2)Ϊԭ�������ߴ�������������������
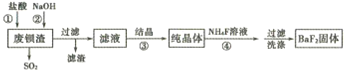
��֪:Kap(BaS2O3)=6.96��10-11��Kap(BaF2)=1.0��10-6
(1)����ٳ�����SO2�������е���ɫ�������ɣ��÷�Ӧ�����ӷ���ʽΪ______________��
(2)����ڵ�Ŀ�����к���������������NaOH��Һ���˹�������ԭ����__________(�����ӷ���ʽ��ʾ)��
(3)��Һ����Ҫ�ɷ���BaCl2������������NaCl���ܽ���������±���
�¶� | 20�� | 40�� | 60�� | 80�� | 100�� |
NaCl | 36.0g | 36.6g | 37.3g | 39.0g | 39.8g |
BaCl2 | 35.8g | 40.8g | 46.2g | 52.5g | 59.4g |
������˲���_____ (���������ᾧ���������½ᾧ��)��
(4)��ҵ�Ͽ��ð�ˮ����SO2����ͨ�����ʹ��ת��Ϊ�̬���ʡ���ת�����������뻹ԭ�������ʵ���֮��Ϊ__________��
(5)���������BaF2�ķ�Ӧ����Ϊ____________��
�����÷�Ӧ�¶ȹ��ߣ��������c(F-)���͵�ԭ����__________��
���о��������ʵ�����NH4F�ı������������BaF2�IJ��ʺʹ��ȣ���Ũ��Ϊ0. 1mol��L-1��BaCl2��Һ��0.22 mol��L-1NH4F��Һ����������������Һ��c(Ba2+)=__________ mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�� a��b��c��d�����ֶ�����Ԫ�ء�a��b��dͬ���ڣ�c��dͬ���塣a��ԭ�ӽṹʾ��ͼΪ
 �� b��c�γɻ�����ĵ���ʽΪ
�� b��c�γɻ�����ĵ���ʽΪ![]() �����бȽ�����ȷ���ǣ� ��
�����бȽ�����ȷ���ǣ� ��
A. ԭ�Ӱ뾶��a��c��d��b B. �縺��a��b��d��c
C. ԭ��������d��a��c��b D. ��ۺ����������c��d��a
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����������غͶ���������ȡ�����ķ�Ӧ����ʽΪ2KClO3![]() 2KCl��3O2����
2KCl��3O2����
�ش��������⣺
��1���÷�Ӧ�б�������Ԫ�ص�����Ϊ____������1 mol O2ʱת�Ƶ��ӵ���Ŀ��________��
��2���ӷ�Ӧ��Ĺ��������з����������ˮ��MnO2�ľ���ʵ��������ƣ�________��
��3���������MnO2������ʵ������ȡCl2����ѧ����ʽΪMnO2��4HCl(Ũ)![]() MnCl2��Cl2����2H2O�������ӷ���ʽΪ________________________________��
MnCl2��Cl2����2H2O�������ӷ���ʽΪ________________________________��
��4����������Ӧ��ת�Ƶĵ��ӵ����ʵ�����ͬ�������ɵ�O2��Cl2����ͬ״���µ������Ϊ________��
��5����˫���ŷ�����MnO2��4HCl(Ũ)![]() MnCl2��Cl2����2H2O����ת�Ƶķ������Ŀ_______��
MnCl2��Cl2����2H2O����ת�Ƶķ������Ŀ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣���ش��й����⣺
| ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | |||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� |
��1�����л�ѧ��������õ�Ԫ�أ���ԭ�ӽṹʾ��ͼΪ _________________________ ��
��2���������γ��������������Ԫ���� __________ ����Ԫ�ط��ű�ʾ����д����Ԫ���������������ˮ���ﷴӦ�Ļ�ѧ����ʽ ________________________________ ��
��3����Ԫ�����Ԫ���γɻ�����ĵ���ʽ _________________________ ��
��4��������������������Ԫ�ص�����������ˮ������������ǿ���� ___________���ѧʽ����
��5����Ԫ�����Ԫ�����ߺ˵����֮���� ____________ ��
��6�����ʵ�鷽�����ȽϢ���ⵥ�������Ե�ǿ�����뽫���������±���
ʵ�鲽�� | ʵ����������� |
______________ | ________________ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com