
����Ŀ����Ӧ4A(s)��3B(g)![]() 2C(g)��D(g)��2 min B��Ũ�ȼ���0.6 mol��L��1�������й�˵����ȷ����
2C(g)��D(g)��2 min B��Ũ�ȼ���0.6 mol��L��1�������й�˵����ȷ����
����A��ʾ�ķ�Ӧ������0.4 mol��L��1��min��1
����2 minĩʱ����Ӧ��B�ķ�Ӧ������0.3 mol��L��1��min��1
������2 min����C��ʾ��ƽ������Ϊ0.2 mol��L��1��min��1
����2 minʱ��B��C��D��Ũ�ȱ�һ��Ϊ3��2��1
����D����ʼŨ��Ϊ0.1 mol��L��1����2 minʱD��Ũ��Ϊ0.3 mol��L��1
A. �٢ڢ�B. �ڢ�C. �ܢ�D. �ۢ�
���𰸡�D
��������
��Aʹ���壬Ũ���dz����������õ�λʱ����Ũ�ȵı仯������ʾ���ٴ���
��B��2min�ڵ�ƽ����Ӧ����0.3mol/��L![]() min����2minĩ�ķ�Ӧ������˲ʱ���ʣ���0.3mol/��L
min����2minĩ�ķ�Ӧ������˲ʱ���ʣ���0.3mol/��L![]() min��С���ڴ���
min��С���ڴ���
��v(C)=![]() v��B��=0.2mol/��L
v��B��=0.2mol/��L![]() min��������ȷ��
min��������ȷ��
�ܲ�֪�����ʵ���ʼŨ�ȣ���������Ũ���뷽��ʽ�Ļ�ѧ������û�б�Ȼ��ϵ���ܴ���
�ݾ�2 min B��Ũ�ȼ���0.6 mol��L��1��B��ϵ��Ϊ3��D��ϵ��Ϊ1������������0.2 mol��L��1��D������2 minʱD��Ũ��Ϊ0.3 mol��L��1������ȷ�����Ԣۢ���ȷ��
��ѡD��


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ţ���е����������ʳ�����ٽ�θҺ���ڡ���ǿ��θ���������ܣ���������б������ã�������ӵĽṹ��ʽΪ![]() ����ҵ�Ͽ�����ϩ���ϳ����ᣬ�������£�
����ҵ�Ͽ�����ϩ���ϳ����ᣬ�������£�
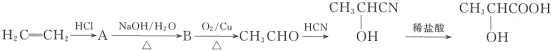
��1�����������Ĺ����ŵ�������______��
��2��д����������ת���Ļ�ѧ����ʽ��
��A��B______��
��![]() __________��
__________��
��3��A��һ�������¿ɷ�����ȥ��Ӧ��д���䷢����ȥ��Ӧ�Ļ�ѧ����ʽ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��E�Ǿ��й�����ζ��Һ�塣A��B��C��D��E��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���
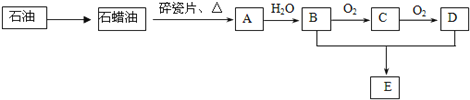
��ش��������⣺
��1����ҵ�ϣ���ʯ�ͻ��ʯ���͵ķ�����___________________��
��2����������ʯ���ͻ��A�Ĺ����е��м����֮һ������һ��ͬ���칹���к�������������CH3����������ͬ���칹��Ľṹ��ʽ�ǣ�___________________��D�����й����ŵ�������_______________��
��3��A��B��0.1 mol����ȫȼ������O2�������_______����״���£���
��4����ӦB��C�Ļ�ѧ����ʽΪ______________________��
��5����ӦB��D��E�Ļ�ѧ����ʽΪ______________________���÷�Ӧ�����ʱȽϻ�����ʵ����Ϊ����߸÷�Ӧ�����ʣ�ͨ����ȡ�Ĵ�ʩ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������Ҫ0.4 mol��L-1NaOH��Һ450 mL��
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����),����������Һ�����õ��IJ���������____________(����������)��

(2)���в�����,����ƿ�����߱��Ĺ�����______________(�����)��
A������һ�����ȷŨ�ȵı���Һ
B��������Һ
C����������ƿ������µ����������Һ��
D��ȷϡ��ijһŨ�ȵ���Һ
E. ���������ܽ��������
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ _________g����ʵ����������������ȷ,������ƿ������ˮϴ�Ӻ�û�и���Ϳ�ʼ��Һ����������ҺŨ�� _____ 0.4 mol��L-1(����������������������С����,��ͬ)����NaOH��Һ��ת��������ƿʱδ����ȴ,��������ҺŨ��_____0.4mol��L-1��������ʱ���ӿ̶��ߣ���������ҺŨ��_____0.4mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ��������������Ƶú�ʹ�ý���п�Ĺ��ң�һ������п��ZnS������SiO2������FeS��CdS��PbS���ʣ�Ϊԭ���Ʊ�����п��������ͼ��ʾ��

��ؽ�������[c0(Mn+)=0.1 mol��L-1]�γ��������������pH��Χ���£�
�������� | Fe3+ | Fe2+ | Zn2+ | Cd2+ |
��ʼ������pH | 1.5 | 6.3 | 6.2 | 7.4 |
������ȫ��pH | 2.8 | 8.3 | 8.2 | 9.4 |
�ش��������⣺
��1�����չ�������Ҫ��Ӧ�Ļ�ѧ����ʽΪ_______________________��
��2������1����Ҫ�ɷֳ�SiO2���___________���������ӹ�����ZnO��������____________������ͨ��������������________________��
��3����Һ�е�Cd2+����п�۳�ȥ����ԭ���ӹ����з�Ӧ�����ӷ���ʽΪ_________________��
��4���������п��Һ�Ʊ�����пʱ�������ĵ缫��ӦʽΪ______________������п��ĵ��Һ�ɷ���_______�������ʹ�á�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��K3[Fe(C2O4)3]��3H2O�������������أ�Ϊ����ɫ���壬������ɹ����ͼ���ش��������⣺
��1��ɹ����ͼʱ����K3[Fe(C2O4)3]��3H2O���й������K3[Fe(CN)6]��ҺΪ��ɫ�������ⷴӦ�Ļ�ѧ����ʽΪ��2K3[Fe(C2O4)3]![]() 2FeC2O4+3K2C2O4+2CO2������ɫ��Ӧ�Ļ�ѧ����ʽΪ______________��
2FeC2O4+3K2C2O4+2CO2������ɫ��Ӧ�Ļ�ѧ����ʽΪ______________��
��2��ijС��Ϊ̽�������������ص��ȷֽ�������ͼ��ʾװ�ý���ʵ�顣
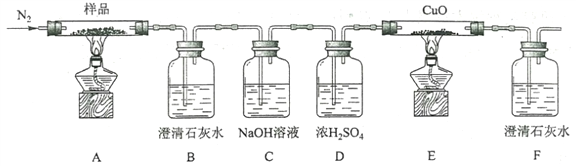
��ͨ�뵪����Ŀ����________________________________________��
��ʵ���й۲쵽װ��B��F�г���ʯ��ˮ������ǣ�װ��E�й����Ϊ��ɫ���ɴ��ж��ȷֽ������һ������___________��___________��
��Ϊ��ֹ������ֹͣʵ��ʱӦ���еIJ�����_____________________________��
����Ʒ��ȫ�ֽ��װ��A�еIJ����ﺬ��FeO��Fe2O3������Fe2O3���ڵķ����ǣ�________________��
��3���ⶨ�����������������ĺ�����
�ٳ���m g��Ʒ����ƿ�У��ܽ���ϡH2SO4�ữ����c mol��L-1 KMnO4��Һ�ζ����յ㡣�ζ��յ��������___________________________��
����������Һ�м������п������Ӧ��ȫ���ˡ�ϴ�ӣ�����Һ��ϴ��Һȫ���ռ�����ƿ�С���ϡH2SO4�ữ����c mol��L-1 KMnO4��Һ�ζ����յ㣬����KMnO4��ҺV mL���þ������������������ı���ʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�Ǿ����Դ���⣬�Ӻ�ˮ����ȡʳ�κ���Ĺ������£�

��1�����оٺ�ˮ���������ַ�����________��________��
��2����������ѻ��Br2�����������ֽ�Br2��ԭΪBr������Ŀ����_________��
��3����������SO2ˮ��Һ����Br2�������ʿɴ�95%���йط�Ӧ�����ӷ���ʽΪ_______���ɴ˷�Ӧ��֪�������������⣬�ڹ�ҵ������Ӧ�������Ҫ������_______��
��4��ij��ѧ�о���ѧϰС��Ϊ���˽�ӹ�ҵ�����ᴿ��ķ������������й�����֪��Br2�ķе�Ϊ59 ��������ˮ���ж�����ǿ��ʴ�ԡ����Dzι��������̺�������װ�ü�ͼ��

�������������ۣ�
��ͼ������B��������____________��
������ʵ��װ�����������Ӿ��������������ܣ���ԭ����__________��
��ʵ��װ�����������ã�Ҫ�ﵽ�ᴿ���Ŀ�ģ���������ο��ƹؼ�������___________��
��C��Һ����ɫΪ________________��Ϊ��ȥ�ò������Բ���������Cl2���������м���NaBr��Һ����ַ�Ӧ���ٽ��еķ��������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʼ�������26 ����������˰����ӵ�����(NA��6.02214076��1023 mol�D1), ������2019 �� 5��20 ����ʽ��Ч������˵������ȷ����
A. �� 1 mol![]() �У����е�������Ϊ 6 NA
�У����е�������Ϊ 6 NA
B. ��7.1 g C12����ˮ�Ƴɱ�����ˮ��ת�Ƶĵ�����Ϊ 0.1 NA
C. ��״���£�11.2 L NO��11. 2 L O2��Ϻ�����ķ�������Ϊ 0.75 NA
D. ij�¶��£�1L pH= 3�Ĵ�����Һϡ�͵�10L ʱ����Һ�� H+����Ŀ����0.01 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����ѧ��ѧ�г������ʵ�ת����ϵ���������ʺͷ�Ӧ������ȥ��
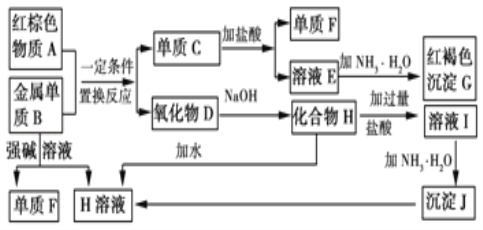
��ش��������⣺
(1)����A�Ļ�ѧʽΪ_____��
(2)д������B��ǿ����Һ��Ӧ�����ӷ���ʽ_____��������D��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ_____��
(3)��ҺE�м��백ˮʱ�������ɰ�ɫ����L��L���ձ�Ϊ���ɫ����G��д��L��ΪG�Ļ�ѧ��Ӧ����ʽ_____��
(4)��ҺE���ڷ������ױ��ʣ�д��������ҺE�Ƿ���ʵ�ʵ�������������_____��Ϊ�˷�ֹ��ҺE�ı��ʣ�������Һ�м���_____�������ӷ���ʽ˵��______________
(5) ��3.06 g����þ�Ļ�����ĩ����100 mL������,ǡ����ȫ��Ӧ,���õ���״����3.36 L H2����úϽ����������ʵ���_____,��Ӧ����Һ��Cl-�����ʵ���Ũ��_____(�ٶ���Ӧ�����Ϊ100 mL)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com