
����Ŀ��A��һ����Ҫ�Ļ���ԭ�ϣ�A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��E�Ǿ��й�����ζ��Һ�塣A��B��C��D��E��һ�������´�������ת����ϵ�����ַ�Ӧ���������ﱻʡ�ԣ���
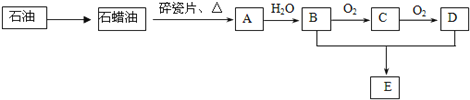
��ش��������⣺
��1����ҵ�ϣ���ʯ�ͻ��ʯ���͵ķ�����___________________��
��2����������ʯ���ͻ��A�Ĺ����е��м����֮һ������һ��ͬ���칹���к�������������CH3����������ͬ���칹��Ľṹ��ʽ�ǣ�___________________��D�����й����ŵ�������_______________��
��3��A��B��0.1 mol����ȫȼ������O2�������_______����״���£���
��4����ӦB��C�Ļ�ѧ����ʽΪ______________________��
��5����ӦB��D��E�Ļ�ѧ����ʽΪ______________________���÷�Ӧ�����ʱȽϻ�����ʵ����Ϊ����߸÷�Ӧ�����ʣ�ͨ����ȡ�Ĵ�ʩ��__________________��
���𰸡����� ![]() �Ȼ� 6.72L 2CH3CH2OH+O2
�Ȼ� 6.72L 2CH3CH2OH+O2![]() 2CH3CHO+2H2O CH3COOH+CH3CH2OH
2CH3CHO+2H2O CH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O ����Ũ���������������ȵ�
CH3COOCH2CH3+H2O ����Ũ���������������ȵ�
��������
A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��A����ϩ��E�Ǿ��й�����ζ��Һ�壬E��������������B���Ҵ���C����ȩ��D�����ᡣ�ݴ˽��
��1����ҵ�ϣ�ʯ��ͨ��������ʯ���͡�
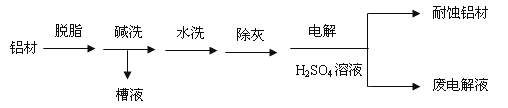
��2������ķ���ʽ��C4H10,����һ��ͬ���칹���к�������������CH3����������ͬ���칹��Ľṹ��ʽ��![]() �� D��CH3COOH�������ŵ��������Ȼ���
�� D��CH3COOH�������ŵ��������Ȼ���
��3��A����ϩ��ȼ�յķ���ʽ��C2H4+3O2��2CO2+2H2O��B���Ҵ���ȼ�յķ���ʽ��C2H6O+3O2��2CO2+3H2O������0.1 mol��ϩ���Ҵ��Ļ������ȫȼ������0.3mol O2�������6.72L��
��4���Ҵ���ͭ�������������±���������Ϊ��ȩ����Ӧ�Ļ�ѧ����ʽΪ2CH3CH2OH+O2![]() 2CH3CHO+2H2O��
2CH3CHO+2H2O��
��5���Ҵ������ᷢ��������Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH2OH![]() CH3COOCH2CH3+H2O���÷�Ӧ�����ʱȽϻ�����ʵ����Ϊ����߸÷�Ӧ�����ʣ�ͨ����ȡ�Ĵ�ʩ�У�����Ũ���������������ȵȡ�
CH3COOCH2CH3+H2O���÷�Ӧ�����ʱȽϻ�����ʵ����Ϊ����߸÷�Ӧ�����ʣ�ͨ����ȡ�Ĵ�ʩ�У�����Ũ���������������ȵȡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���㡢Ϻ������С�ӱ������������ס��ҡ�������(��ͼ)�������ų��ķ�Һ�ÿ������ֻ�� Na2CO3��CuCl2��Ca(OH)2��HCl �е�һ�֡�ij��ѧ����С��Ժ�ˮ���ʱ���֣��� �״���ˮ�����ҳ���ɫ���� �Ҵ���ˮ�����ɫ���DZ�dz��ɫ���ǣ��� ������ˮ�ɻ���壻�� �����������ݣ���ˮ���壬���ƶϣ�
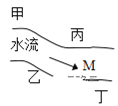
(1)���������ų��ķ�Һ�ﺬ�е���Ⱦ���_______����________����_______����________��
(2)��M��ȡ���ĺ�ˮ�У��϶����е�������____________________��
(3)д�����������ܷ��������ӷ���ʽ______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��D��E��W����ѧ����������Ԫ�أ�ԭ��������������A��ԭ�������������Ǵ�����2����D���������������������D��Eλ��ͬ���ڣ�A��D��E��ԭ������������֮��Ϊ14��W������������Ԫ�أ�ȱW�ᵼ��ƶѪ֢״��
��1��д��AE4�ĵ���ʽ��____________________��
��2��������ʵ����Ԫ�������ɽ��͵��ǣ�����ĸ��ţ�___________��
a��D������������Ӧˮ����ļ�������Mg(OH)2
b��E����̬�⻯����ȶ���С��HF
c��WE3����Һ�����ڿ�ʴͭ�Ƶ�ӡˢ��·��
��3��NaCN��һ���о綾���Σ���E��һ��������EO2���Գ�ȥˮ��Һ�к��еĸ��ж����ʣ��õ�һ�������г����Ĺ�������������塣д���÷�Ӧ�����ӷ���ʽ��_________________________________________��
��4����ҵ���õ�ⷨ�Ʊ�D�ĵ��ʣ���Ӧ�Ļ�ѧ����ʽΪ_____________________��
��5��W�ĵ��ʿ����ڴ������Է�ˮ�е�NO3-��ʹ��ת��ΪNH4+��ͬʱ�����д��Ե�W��������X���ٽ��к���������
��������Ӧ�����ӷ���ʽΪ___________________________________________��
��D�ĵ�����X�ڸ����·�Ӧ�Ļ�ѧ����ʽΪ____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����������������
A.��0.1 mol��L��1�Ĵ�����Һ�м�ˮ��ͨ��HCl���嶼��ʹ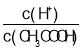 ֵ����
ֵ����
B.0.1 mol��L��1�Ĵ�������Һ20 mL��0.1 mol��L��1����10 mL��Ϻ���Һ�����ԣ�����c(CH3COO��)��c(Cl��)��c(H��)��c(CH3COOH)
C.pH��4�Ĵ�����pH��10������������Һ�������ϣ�������ҺpH��7
D.0.1 mol��L��1ijһԪ��HA��Һ��![]() ��1��10��8�������Һ����ˮ�������c(H��)��1��10��11mol��L��1
��1��10��8�������Һ����ˮ�������c(H��)��1��10��11mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������п��ŨH2SO4����ڼ����·�Ӧ���ɻ�SO2��H2�Ļ�����壻п��ϡ���ᷴӦֻ��H2���ɡ���֪��Zn+2H2SO4(Ũ)![]() ZnSO4+2H2O+SO2�������м������о�С��ֱ�ʵ��̽����
ZnSO4+2H2O+SO2�������м������о�С��ֱ�ʵ��̽����
��1�����о�С�鰴��ͼʵ����֤п��Ũ���ᷴӦ��������SO2��H2��ȡ������Zn����b�У���a�м���100mL18.5mol��L��1��Ũ���ᣬ����һ��ʱ�䷴Ӧ��Zn��ȫ�ܽ⡣
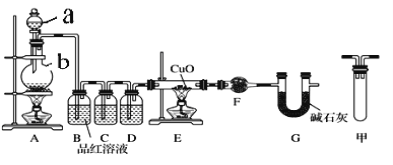
����д�������ƣ�a___________��b___________��
���о�С����Ϊ�����ܲ��������������ǣ�_____________________��
��װ��D�м�����Լ���__________��
��U��G������Ϊ__________��
����ͬѧ��ΪA��B��Ӧ����ͼ�еļ�װ�ã���װ�õ�����Ϊ__________��
��֤����Ӧ����SO2��H2��ʵ��������______________________________��
��2�����о�С��Ϊ��̽��п��ϡ���ᷴӦ�����е����ʼ������ı仯����������ʵ�飬����Ӱ�췴Ӧ���ʵ����ء�

ʵ��ʱ���ӶϿ�K��ʼ��ÿ���1���ӣ�����Ͽ���պ�K������������ÿ1�����ڴ�a��������ˮ�������õ���ˮ�������±���ʾ��
1����ˮ�������Ͽ�K�� | 34 | 59 | 86 | 117 | �� | 102 |
1����ˮ�������պ�K�� | 58 | 81 | 112 | 139 | �� | 78 |
������Ӧ�����е�ˮ��������ش�
�� ��ˮ����58��34��81��59��˵���ڷ�Ӧ���ڣ��պ�Kʱ�ȶϿ�Kʱ�ķ�Ӧ���ʿ죬��Ҫԭ����________��
�� ��ˮ����102��78��˵���ڷ�Ӧ���ڣ��Ͽ�Kʱ�ķ�Ӧ���ʿ��ڱպ�Kʱ�ķ�Ӧ���ʣ���Ҫԭ����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Խ�����Ʒ���и㸯ʴ���������ӳ���ʹ��������
��1������Ϊ������洦����һ�ַ�����
����ϴ��Ŀ���dz�ȥ����������Ȼ����Ĥ����ϴ��ʱ��������ð����ԭ���ǣ�________�������ӷ���ʽ��ʾ����Ϊ����ϴ��Һ�е����Գ�����ʽ���գ�������Һ�м��������Լ��е�__________��
a.NH3b.CO2c.NaOH d.HNO3
��������Ϊ��������H2SO4��Һ�е�⣬��������γ�����Ĥ�������缫��ӦʽΪ��_____��
ȡ�����ϵ��Һ������NaHCO3��Һ��������ݺͰ�ɫ����������������ԭ����_________��
(2)��ͭ�ɷ�ֹ����Ʒ��ʴ�����ʱ��ͭ������ʯī��������ԭ���� ��
��3��������ͼװ�ã�����ģ�����ĵ绯ѧ������

��XΪ̼����Ϊ�������ĸ�ʴ������KӦ������ ����
��XΪп������K����M�����õ绯ѧ��������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(DMP)��һ���ܻ�������Է�������Ϊ194�����˺��������һ����Σ������ṹ�ɱ�ʾΪ![]() (����RΪ����)ʵ���������·����ɵõ�DMP��
(����RΪ����)ʵ���������·����ɵõ�DMP��
![]()
��ش�
(1)����C�й����ŵ�����Ϊ_____��
(2)�����й�DMP��˵��������ȷ����_______��(����ĸ���)
A��DMP�ķ���ʽΪC10H12O4
B��DMP���Է���ȡ�����ӳɡ������ȷ�Ӧ
C��DMP��ˮ�е��ܽ�Ȳ���
(3)B���Ҷ���(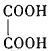 )��һ�������¿������ʵ���1��1������Ӧ���ɸ߷��ӻ�����ø߷��ӻ�����Ľṹ��ʽΪ______��
)��һ�������¿������ʵ���1��1������Ӧ���ɸ߷��ӻ�����ø߷��ӻ�����Ľṹ��ʽΪ______��
(4)��ҵ�����ڶ��ױ�(![]() )Ϊԭ������������(
)Ϊԭ������������( )����ʹ����ij����һ�������·�Ӧ��ȡDMP��������ô���ȡDMP�Ļ�ѧ����ʽΪ________��
)����ʹ����ij����һ�������·�Ӧ��ȡDMP��������ô���ȡDMP�Ļ�ѧ����ʽΪ________��
(5)���㻯����E��C��Ϊͬ���칹�壬��lmo E������������Һ��Ӧ�������2mol Ag����E���ܵĽṹ��ʽΪ______��BҲ���ڶ���ͬ���칹�壬��������������B��ͬ���칹����______�֡�
��l mol�л��������2mol NaOH��Ӧ
�ڱ����ϵ�һ�ȴ���ֻ��һ�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ӧ4A(s)��3B(g)![]() 2C(g)��D(g)��2 min B��Ũ�ȼ���0.6 mol��L��1�������й�˵����ȷ����
2C(g)��D(g)��2 min B��Ũ�ȼ���0.6 mol��L��1�������й�˵����ȷ����
����A��ʾ�ķ�Ӧ������0.4 mol��L��1��min��1
����2 minĩʱ����Ӧ��B�ķ�Ӧ������0.3 mol��L��1��min��1
������2 min����C��ʾ��ƽ������Ϊ0.2 mol��L��1��min��1
����2 minʱ��B��C��D��Ũ�ȱ�һ��Ϊ3��2��1
����D����ʼŨ��Ϊ0.1 mol��L��1����2 minʱD��Ũ��Ϊ0.3 mol��L��1
A. �٢ڢ�B. �ڢ�C. �ܢ�D. �ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Na2SO4����������480mL0.2mol��L-1��Na2SO4��Һ���ɹ�ѡ���������ͼ��
��1����ͼ��ʾ��������������Һ����Ҫ����__ (��ѡ��)������������Һ�����õ��IJ���������___��__ (����������)��
��2��ʹ������ƿ֮ǰ������еIJ�����___��(��ѡ��)
A.��������� B.����Ƿ�©ˮ C.���
��3�������㣬��Na2SO4������Ϊ___g��
��4����ѡ�õ�����ƿ���Ϊ___mL��
��5��������Һʱ��һ��ɷ�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶���
�����˳����__��__��__��__��__��__(�����)��___
��6�������ƹ����У�����������ȷ������ʱ���ӿ̶���ʹ������ҺŨ��__(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com