
����Ŀ������������Ͷ���������������������Ҫ���ʣ���ҵ�ö��ַ�����������ij���ۺϴ�����NH4����ˮ��ҵ����(��Ҫ��NO��CO��CO2��SO2��N2)��������ͼ��
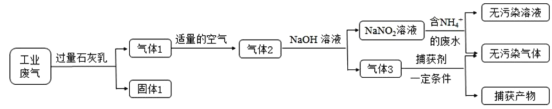
��֪��NO+NO2+2NaOH=2NaNO2+H2O 2NO2+2NaOH=NaNO3+NaNO2+H2O
(1)����1����Ҫ�ɷ���Ca(OH)2��_______(�ѧʽ)��
(2)��NaNO2��Һ������NH4����ˮ��Ӧ�����ӷ���ʽΪ____��
(3)��֤��ˮ��NH4���ѻ��������ķ�����________(д�����������������)��
(4)����1ת��Ϊ����2ʱ�������ܹ�����ԭ����_________��
(5)����������������Ҫ��__________(�ѧʽ)��
(6)���������ɵ�NaNO2����ۺ�ʳ�����ƣ�������ζ������ʹ����ʳ�ж�����֪NaNO2�ܷ������·�Ӧ��2NaNO2+4HI=2NO��+I2+2NaI+2H2O��I2����ʹ���۱���������������Ӧ��ѡ�������г��������ʺ��й��Լ�����ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ�������____(�����)��
��ˮ �ڵ��۵⻯����ֽ �۵��� �ܰ� �ݰ״�
A���٢ۢ� B���٢ڢ� C���٢ڢ� D���٢ڢۢ�
���𰸡�CaCO3��CaSO3 NH4++NO2-=N2��+2H2O ȡ�����������ˮ���Թ��У�����NaOH��Һ���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ����������������֤��NH4���ѻ������� ����1ת��Ϊ����2ʱ��ֻ�е�����NO��NO2���ʵ���֮��Ϊ1��1ʱ���ſ��Ա�NaOH��Һ��ȫת����NaNO2������������������NaOH��Ӧ����NaNO3��NaNO2�Ļ����Һ����˿������ܹ��� CO C
��������
��ҵ������CO2��SO2�ɱ�ʯ�������գ������ΪCaCO3��CaSO3������Ca(OH)2��������Dz��ܱ�����ʯ��ˮ���յ�N2��NO��CO��������������Ŀ������÷�Ӧ����NO2������NaOH��Һ�����õ�NaNO2���������ܹ���������õ�NaNO3���ɢ�2NO+O2=2NO2����NO2+NO+2NaOH=2NaNO2+H2O������+����2�õ�4NO+O2+4NaOH=4NaNO2+4H2O��Ϊȷ����Ӧֻ����NaNO2��������Ӧ����NO��O2���ʵ���֮��4��1������3����CO��N2��NaNO2�뺬��NH4+����Һ��Ӧ��������Ⱦ��N2��������������������Ҫ��CO�����ݵ�����Һ��I2��Ϊ��ɫ����NaNO2�Ĵ��ڡ�
(1)��ҵ������CO2��SO2����������������Ca(OH)2������Ӧ������ɱ�ʯ�������շ�Ӧ������Ӧ���Σ����Թ����ΪCaCO3��CaSO3������Ca(OH)2��
(2)��NaNO2���������ԣ�NH4+���л�ԭ�ԣ���������Һ�з���������ԭ��Ӧ����N2��H2O��������NaNO2��Һ������NH4����ˮ��Ӧ�����ӷ���ʽΪ��NH4++NO2-=N2��+2H2O��
(3)NH4+�ܹ���ǿ��Ȳ��������������������������֤��ˮ��NH4+�ѻ��������ķ����ǣ�ȡ�����������ˮ���Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ����������������֤��NH4���ѻ���������
(4)������������NO�ᱻ��ȫ��������NO2��ֻ������Ӧ2NO2+2NaOH=NaNO3+NaNO2+H2O��ʹ��ȡ�õ��������к���NaNO3���ʣ����ݷ���ʽNO+NO2+2NaOH=2NaNO2+H2O��֪��ֻ�е�������NO��NO2���ʵ���֮��Ϊ1��1ʱ�ſ��Ա�NaOH��Һ��ȫת����NaNO2������ͨ��Ŀ������ܹ�����
(5)����3����CO��N2��NaNO2�뺬��NH4+����Һ��Ӧ��������Ⱦ���壬���ɵĸ�����ӦΪN2��������������������Ҫ��CO��
(6)���ݷ�Ӧ����ʽ2NaNO2+4HI=2NO��+I2+2NaI+2H2O��֪��NaNO2���������ԣ��ὫKI��������I2��I2����ʹ���۱�������Ӧ��������Һ�н��У���Ӧ����Һ�н��У���Ҫˮ���ᣬ����ҪKI�����ۣ����Լ���NaNO2��NaCl��Ҫ���Լ����Ϊ�٢ڢݣ��ʺ���ѡ����C��


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���Բ����ƿ�м���1 molC2H5OH�ͺ�1molHBr�������ᣬ��Һ�з�����Ӧ��C2H5OH+HBr![]() C2H5Br+H2O����ַ�Ӧ��ﵽƽ�⡣��֪��ѹ�£�C2H5Br��C2H5OH�ķе�ֱ�Ϊ38.4���78.5�档�����й�����������ǣ� ��
C2H5Br+H2O����ַ�Ӧ��ﵽƽ�⡣��֪��ѹ�£�C2H5Br��C2H5OH�ķе�ֱ�Ϊ38.4���78.5�档�����й�����������ǣ� ��
A. ����NaOH��Һ���������Ҵ������ʵ���
B. �����������Ũ�ȣ�����������C2H5Br
C. ����Ӧ���������2 mol�������ַ�Ӧ��ƽ��ת����֮�ȱ��
D. ����ʼ�¶������60�棬���������C2H5Br�IJ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ķ�Ӧ·��������Ϣ��գ�
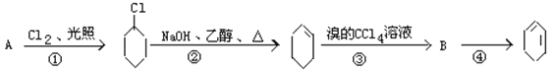
��1��A�Ľṹ��ʽ��_____________��������_______________________��
��2���ٵķ�Ӧ����______________���ڵķ�Ӧ����__________________��
��3����Ӧ�ڵĻ�ѧ����ʽ_______________________________��
��Ӧ�ܵĻ�ѧ����ʽ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ӦA(g) + 3B(g)![]() 2C(g) + D(g)������ijʱ�������A��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ����Ϊ1mol��L-1��min-1�����ڴ�ʱ�������C��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ����Ϊ
2C(g) + D(g)������ijʱ�������A��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ����Ϊ1mol��L-1��min-1�����ڴ�ʱ�������C��Ũ�ȱ仯��ʾ�Ļ�ѧ��Ӧ����Ϊ
A. 0.5 mol��L-1��min-1B. 2 mol��L-1��min-1
C. 3 mol��L-1��min-1D. 1 mol��L-1��min-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���X��һ����Ҫ���л�����ԭ�ϣ���ͼ������Ϊ��ʼԭ����Ƴ���ת����ϵͼ(���ֲ���ϳ�·�ߡ���Ӧ������ȥ)��Y��һ�ֹ��ܸ߷��Ӳ��ϡ�
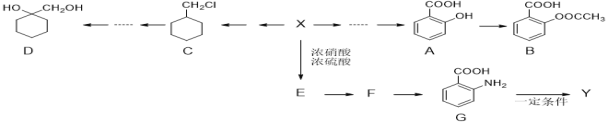
��֪��
(1)XΪ������������Է�������Ϊ92��
(2)������ڸ�����ص������£��������������Ȼ���![]() ��
��
(3)![]() (�������ױ�����)��
(�������ױ�����)��
�ش��������⣺
(1)X������Ϊ________________��G�ķ���ʽΪ____________________��
(2)F�Ľṹ��ʽΪ___________________��B�й����ŵ�����Ϊ____________��
(3)X��E�ķ�Ӧ����Ϊ__________________________��
(4)A��B�Ļ�ѧ����ʽΪ__________________________��
(5)��������������B��ͬ���칹����________�֡�
�ٺ��б�������ֻ��һ�ֹ����ţ���1 mol���л�������2 mol NaHCO3��ȫ��Ӧ��
(6)��д����CΪԭ���Ʊ�![]() �ĺϳ�·������ͼ(���Լ�����)_________��
�ĺϳ�·������ͼ(���Լ�����)_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ƥ�������������״̬ʱ�ṹ��ͼ����������������з�������25����101 kPaʱ�����й��̵��ʱ䡢�ر���Է�������Ƥ�������״̬������״̬һ�µ��ǣ���

A. CaCO3=CaO+CO2�� B. NaOH���ܽ�
C. 2H2+O2=2H2O D. Ba(OH)2��8H2O+2NH4Cl=BaCl2+2NH3��+10H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ֲ��֦Ҷ��ȡ�ľ����к������мס������ֳɷ֣�
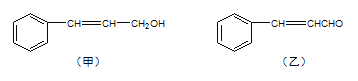
���������գ�
��1�����еĺ��������ŵĵ���ʽΪ____�����к��������ŵ�����Ϊ____��
��2���ɼ�ת��Ϊ���辭���й��̣�
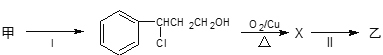
���з�Ӧ��ķ�Ӧ����Ϊ___����Ӧ��Ļ�ѧ����ʽΪ___����Ӧ����Ϊ___����Ʒ�Ӧ���Ŀ����___��
��3�����������е�̼̼˫������ѡ�õ��Լ���____����ѡ����ѡ���ԭ��____��
a����ˮ b�����Ը��������Һ c�����CCl4��Һ d��������Һ
��4���Ҿ����⻯�������õ�����![]() �������ж���ͬ���칹�壬д�����ϱ�������һ��ȡ���������������ͬ���칹��____��д�������ܷ���������Ӧ��һ�����ڼ��������µ�ˮ�ⷴӦ�Ļ�ѧ����ʽ____��
�������ж���ͬ���칹�壬д�����ϱ�������һ��ȡ���������������ͬ���칹��____��д�������ܷ���������Ӧ��һ�����ڼ��������µ�ˮ�ⷴӦ�Ļ�ѧ����ʽ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����P��S��Cl��Ni��Ԫ����ɵ����Ͳ������Ź㷺����;���ش��������⡣
��1����̬Clԭ�Ӻ�������Ų�ʽΪ____��P��S��Cl�ĵ�һ�������ɴ�С˳��Ϊ____��
��2��SCl2�����е�����ԭ���ӻ����������____���÷��ӹ���Ϊ_____��
��3��Ni��CO���γ������Ni(CO)4���÷�������λ������Ϊ____������������ʾ��������������ʾ��λ����д��Ni(CO)4���ӵĽṹʽ____��
��4����֪MgO��NiO�ľ���ṹ(��ͼ)��ͬ������Mg2+��Ni2+�����Ӱ뾶�ֱ�Ϊ66 pm��69pm�����۵㣺MgO_____NiO(������������������������)��������____��
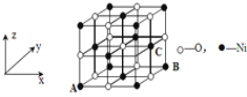
��5����NiO�����������������AΪ(0��0��0)��BΪ(1��1��0)����C�����������Ϊ____��
��6�����ʯ��������___��̼ԭ�ӡ���̼ԭ�Ӱ뾶Ϊr�����ʯ�����ı߳�Ϊa������Ӳ��Ӵ�ģ�ͣ���r= ______a����ʽ��ʾ̼ԭ���ھ����еĿռ�ռ����____������r��a��ʾ��Ҫ�����������
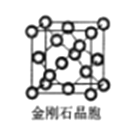
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����51.2g Cu��ȫ��������Ũ�����У��ռ��������������NO��N2O4��NO2���Ļ���ﹲ0.8mol����Щ����ǡ���ܱ�500 mL NaOH��Һ��ȫ���գ�����NaNO2��NaNO3��������Һ������NaNO3�����ʵ���Ϊ0.2mol����NaOH��Ũ��Ϊ �� ��
A.1.8mol/LB.2mol/LC.2.4 mol/LD.3.6 mol/L
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com