
����Ŀ���Ե���Ϊ��Ҫԭ�Ϻϳ�һ�־��й���ζ�л���C�߷��ӻ�����E�ĺϳ�·����ͼ1��ʾ��

��ش��������⣺
(1)E�Ľṹ��ʽΪ________��D�����ں��еĹ�������________(������)��
(2)д����Ӧ�ڵķ�Ӧ���ͣ�________��
(3)д�����з�Ӧ�Ļ�ѧ����ʽ��
��________________________________________________________________________��
��________________________________________________________________________��
(4)ijͬѧ����ͼ2װ���Ʊ�����C���Թ�B��װ�������ı���̼������Һ��Ŀ���ǣ�________________________________________�������Թ�B�ĵ��ܽ���һ����״�������Ϊ________________________________________________________________________�����轫�Թ�B�е�����C����������õ�����Ҫ���������У��ձ���________��

���𰸡�)![]() ̼̼˫�� ������Ӧ 2CH3CH2OH��O2
̼̼˫�� ������Ӧ 2CH3CH2OH��O2![]() 2CH3CHO��2H2O CH3COOH��CH3CH2OH
2CH3CHO��2H2O CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O ���������������ܽ�ȣ����Ҵ������� ������ ��Һ©��
CH3COOCH2CH3��H2O ���������������ܽ�ȣ����Ҵ������� ������ ��Һ©��
��������
CH3CH2OH��Cu���������·�������������AΪCH3CHO��CH3CHO��һ����������BΪCH3COOH��CH3COOH��CH3CH2OH����������Ӧ����CΪCH3COOC2H5���Ҵ�������ȥ��Ӧ����DΪCH2=CH2����ϩ�����Ӿ۷�Ӧ���ɸ߷�������EΪ����ϩ���ݴ˴��⡣
��1�������Ϸ�����֪��EΪ����ϩ���ṹ��ʽΪ��![]() ��DΪ��ϩ���ṹ��ʽΪ��CH2=CH2�����еĹ�����Ϊ̼̼˫�����ʴ�Ϊ��
��DΪ��ϩ���ṹ��ʽΪ��CH2=CH2�����еĹ�����Ϊ̼̼˫�����ʴ�Ϊ��![]() ��̼̼˫����
��̼̼˫����
��2����Ϊ��ȩ��������Ӧ���������ᣬ�ʴ�Ϊ��������Ӧ��
��3����Ϊ�Ҵ��Ĵ�������Ӧ����Ӧ����ʽΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ϊ�������Ҵ���Ũ���������·���������Ӧ����Ӧ����ʽΪ��CH3COOH��CH3CH2OH
2CH3CHO+2H2O����Ϊ�������Ҵ���Ũ���������·���������Ӧ����Ӧ����ʽΪ��CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O���ʴ�Ϊ��2CH3CH2OH+O2
CH3COOCH2CH3��H2O���ʴ�Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O��CH3COOH��CH3CH2OH
2CH3CHO+2H2O��CH3COOH��CH3CH2OH![]() CH3COOCH2CH3��H2O��
CH3COOCH2CH3��H2O��
��4���Թ�B��װ�������ı���̼������Һ��Ŀ���Ǣ����������ڱ���Na2CO3��Һ�е��ܽ�Ƚ�С����С�ܽ⣬���ڷֲ��������ڻӷ�����������Na2CO3��Ӧ���������ᣬ�ۻӷ������Ҵ���Na2CO3��Һ���գ��������������ڱ���̼������Һ�����÷�Һ�ķ������룬�õ����������ձ�����Һ©���ȣ������Թ�B�ĵ��ܽ���һ����״�������Ϊ���������ʴ�Ϊ�����������������ܽ�ȣ����Ҵ����������������Һ©����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������茶���[��NH4��2Fe��SO4��2��6H2O]�Ƿ�����ѧ����Ҫ���Լ��������ڴ���������������������茶�����500��Cʱ��������������ȫ�ֽ⡣�ش��������⣺
��1����������茶����������������ȫ�ֽ⣬������������ԭ��Ӧ��������������FeO��Fe2O3��������������NH3��SO3��H2O��N2��__________________
��2��Ϊ����ֽ����ijɷ֣��������ʵ��װ�ý���ʵ�飬����A�е���������茶������ֽ���ȫ��

��A�й����ּ��Ƚϳ�ʱ���ͨ�뵪����Ŀ����_______________________________��
��Ϊ����A�в������Ƿ���FeO����Ҫѡ�õ��Լ���______________�����ţ���
A.KSCN��Һ B.ϡ���� C.Ũ���� D.KMnO4��Һ
��3��ͨ�����Ը��������Һ����Һ��ɫ���÷�Ӧ��SO2���ֳ���ѧ������_____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��Ӧ��CO(g)��CuO(s)![]() CO2(g)��Cu(s)�ͷ�Ӧ��H2(g)��CuO(s)
CO2(g)��Cu(s)�ͷ�Ӧ��H2(g)��CuO(s)![]() Cu(s)��H2O(g)����ͬ��ij�¶��µ�ƽ�ⳣ���ֱ�ΪK1��K2�����¶��·�Ӧ��CO(g)��H2O(g)
Cu(s)��H2O(g)����ͬ��ij�¶��µ�ƽ�ⳣ���ֱ�ΪK1��K2�����¶��·�Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g)��ƽ�ⳣ��ΪK��������˵����ȷ����(����)
CO2(g)��H2(g)��ƽ�ⳣ��ΪK��������˵����ȷ����(����)
A. ��Ӧ�ٵ�ƽ�ⳣ��K1��c(CO2).c(Cu)/c(CO).c(CuO)
B. ��Ӧ�۵�ƽ�ⳣ��K��K1/K2
C. ���ڷ�Ӧ�ۣ�����ʱ���¶����ߣ�H2Ũ�ȼ�С����÷�Ӧ���ʱ�Ϊ��ֵ
D. ���ڷ�Ӧ�ۣ����º����£�����ѹǿ��H2Ũ��һ����С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͬ��ͬѹ�£���A�����г�������O2��B�����г�������O3
��1������������������ȣ���A������B�������ݻ�֮����____��
��2����������������ԭ��������ȣ���A������B�������ݻ�����____��
��3�����������������Ϊ3��2����O2��O3���ʵ���֮��Ϊ____������֮��Ϊ____���ܶ�֮��Ϊ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и���������ˮ��Һ��һ���ܴ����������(����)
A. ���д��� Ba2������Һ�У�Cl����K����![]() ��
��![]()
B. ���д���H������Һ�У�Mg2����Na����![]() ��
��![]()
C. ���д���OH������Һ�У�Mg2����![]() ��
��![]() ��
��![]()
D. ���д���Na������Һ�У�H����K����![]() ��
��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ����A��F֮���ת����ϵ������AΪ����ɫ�������ʣ�B��CΪ��ɫ��Һ��DΪ���壬E��FΪ��ɫ����������д���и��գ�
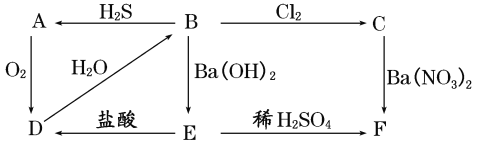
��1��д�������ʵĻ�ѧʽ��
AΪ______��BΪ_____��CΪ_____��DΪ_____��EΪ_____��FΪ______��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
B��A��________________________��
B��C��___________________________��
��3��д��C��F�����ӷ���ʽ��___________________��
��4����A��F���������У��������������л�ԭ�Ե���(����ĸ����)______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[���ʽṹ������]
[Zn(CN)4]2-��ˮ��Һ����HCHO�������·�Ӧ��4HCHO+[Zn(CN)4]2-+4H++4H2O===[Zn(H2O)4]2++4HOCH2CN
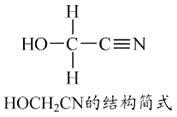
��1��Zn2+��̬��������Ų�ʽΪ____________________��
��2��1 mol HCHO�����к�����������ĿΪ____________mol��
��3��HOCH2CN������̼ԭ�ӹ�����ӻ�������______________��
��4����H2O���ӻ�Ϊ�ȵ������������Ϊ________________��
��5��[Zn(CN)4]2-��Zn2+��CN-��Cԭ���γ���λ���������ǿռ乹�ͣ�[Zn(CN)4]2-�Ľṹ����ʾ��ͼ��ʾΪ_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��[ʵ�黯ѧ]
���������ƣ�Na2S2O5���dz��õĿ����������ڿ����С�����ʱ���ֽ⡣ʵ�����Ʊ�����Na2S2O5�ķ������ڲ��Ͻ����£����Ʒ�Ӧ�¶���40�����ң���Na2CO3��������Һ��ͨ��SO2��ʵ��װ������ͼ��ʾ��

����ҺpHԼΪ4ʱ��ֹͣ��Ӧ����20�����Ҿ��ýᾧ������Na2S2O5�Ļ�ѧ����ʽΪ
2NaHSO3===Na2S2O5+H2O
��1��SO2��Na2CO3��Һ��Ӧ����NaHSO3��CO2�������ӷ���ʽΪ____________________��
��2��װ��Y��������______________________________��
��3����������ķ�ӦҺ����ѹ���ˡ�ϴ�ӡ�25����30������ɻ��Na2S2O5���塣
����ɼ�ѹ����װ�õ���Ҫ�����Dz���©����________________�ͳ����á�
�������ñ���SO2ˮ��Һ����ˮ�Ҵ�ϴ��Na2S2O5���塣�ñ���SO2ˮ��Һϴ�ӵ�Ŀ����______��
��4��ʵ���Ƶõ�Na2S2O5�����к���һ������Na2SO3��Na2SO4������ܵ�ԭ����______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��25 ��ʱ�й�����ĵ���ƽ�ⳣ�����±���
����Ļ�ѧʽ | CH3COOH | HCN | H2C2O4 |
����ƽ�ⳣ�� | 1.8��10��5 | 4.9��10��10 | K1��5.9��10��2��K2��6.4��10��6 |
�����й�˵����ȷ����(����)
A. CH3COOH��Һ��Na2CO3��Ӧ����CO2����֤������������
B. H2C2O4��Һ�ĵ��뷽��ʽΪ H2C2O4![]() 2H+ + C2O42-
2H+ + C2O42-
C. ��ˮϡ��HCN��Һ���ٽ�HCN�ĵ�����c(CN-)/c(OH-)����
D. ��Na2C2O4 ��Һ�м���������CH3COOH��Һ������Ӧ�����ӷ���ʽΪ C2O42- + CH3COOH=CH3COO- + HC2O4-
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com