
ЁОЬтФПЁПвбжЊЃК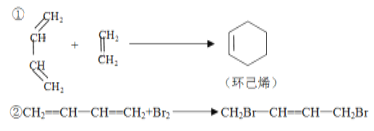
(1)аДГіЯТСаЗДгІВњЮяЕФНсЙЙМђЪНЃК
H2C=CHCH=CHCH3+H2C=CHCHOЁњ _______________ЁЃ
(2)вдФГСДЬўAЮЊЦ№ЪМдСЯКЯГЩЛЏКЯЮяG ЕФТЗОЖШчЯТ(ЭМжа Mr БэЪОЯрЖдЗжзгжЪСП)
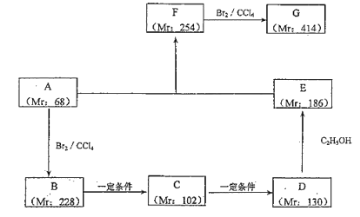
ЂйаДГіЗДгІРраЭ BЁњCЃК_______ЃЌFЁњGЃК_______ЁЃ
ЂкаДГіЯТСаЮяжЪЕФНсЙЙМђЪНЃКAЃК________ЃЌFЃК_______ЁЃ
ЂлаДГіЯТСаЗДгІЕФЛЏбЇЗНГЬЪНЃК
BЁњCЃК________________ЃЛDЁњEЃК_______________ЁЃ
ЂмаДГі G гыЧтбѕЛЏФЦШмвКЗДгІЕФЗНГЬЪН____________________ЁЃ
ЁОД№АИЁП
(1)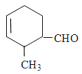 Лђ
Лђ ЃЛ
ЃЛ
(2)ЂйЫЎНтЗДгІ(ШЁДњЗДгІ)ЃЛМгГЩЗДгІЃЛ
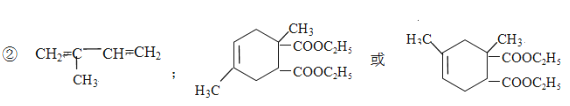 ЃЛ
ЃЛ
ЂлBrCH2-C(CH3)=CH-CH2Br+2NaOH![]() CH2(OH)-C(CH3)=CH-CH2(OH)+2NaBrЃЛ
CH2(OH)-C(CH3)=CH-CH2(OH)+2NaBrЃЛ
HOOC-C(CH3)=CH-COOH+2CH3CH2OH![]() H5C2OOC-C(CH3)=CH-COOC2H5+2H2O
H5C2OOC-C(CH3)=CH-COOC2H5+2H2O
Ђм
ЁОНтЮіЁП
ЪдЬтЗжЮіЃК(1)ИљОнаХЯЂЃЌH2C=CHCH=CHCH3+H2C=CHCHOЁњ Лђ
Лђ ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК Лђ
Лђ ЃЛ
ЃЛ
(2)ФГСДЬўAЕФЯрЖдЗжзгжЪСПЮЊ68ЃЌдђЗжзгжаЬМдзгзюДѓЪ§ФПЮЊ![]() =5Ё8ЃЌЫљвдИУЬўЕФЗжзгЪНЮЊC5H8ЃЌЦфВЛБЅКЭЖШ=
=5Ё8ЃЌЫљвдИУЬўЕФЗжзгЪНЮЊC5H8ЃЌЦфВЛБЅКЭЖШ=![]() =2ЃЌдђИУЬўЪЧШВЬўЛђЖўЯЉЬўЃЛAКЭфхЗЂЩњМгГЩЗДгІЩњГЩBЃЌИљОнAКЭBЕФЯрЖдЗжзгжЪСПжЊЃЌAКЭфхЪЧ1ЃК1МгГЩЃЌдђAЪЧЖўЯЉЬўЃЌСНИіЫЋМќЭЌЪБСЌдквЛИіЬМдзгЩЯВЛЪЧЮШЖЈНсЙЙЃЌдђAЕФНсЙЙМђЪНЮЊЃК
=2ЃЌдђИУЬўЪЧШВЬўЛђЖўЯЉЬўЃЛAКЭфхЗЂЩњМгГЩЗДгІЩњГЩBЃЌИљОнAКЭBЕФЯрЖдЗжзгжЪСПжЊЃЌAКЭфхЪЧ1ЃК1МгГЩЃЌдђAЪЧЖўЯЉЬўЃЌСНИіЫЋМќЭЌЪБСЌдквЛИіЬМдзгЩЯВЛЪЧЮШЖЈНсЙЙЃЌдђAЕФНсЙЙМђЪНЮЊЃК![]() ЃЌBЭЈЙ§ЫЎНтЗДгІЃЌфхдзгБЛШЁДњв§Шы2ИієЧЛљЩњГЩCЃЎИљОнDКЭEЕФЯрЖдЗжзгжЪСППЩХаЖЯЃЌDдкХЈСђЫсМгШШЬѕМўЯТгыввДМЗЂЩњѕЅЛЏЗДгІЩњГЩEЃЌDжаКЌга2ИієШЛљЃЌЙЪBЮЊBrCH2C(CH3)=CHCH2BrЃЌCЮЊHOCH2C(CH3)=CHCH2OHЃЌDЮЊHOOCC(CH3)=CHCOOHЃЌEЮЊCH3CH2OOCC(CH3)=CHCOOCH2CH3ЃЎИљОнвбжЊаХЯЂПЩжЊЃЌFЕФНсЙЙМђЪНЮЊ
ЃЌBЭЈЙ§ЫЎНтЗДгІЃЌфхдзгБЛШЁДњв§Шы2ИієЧЛљЩњГЩCЃЎИљОнDКЭEЕФЯрЖдЗжзгжЪСППЩХаЖЯЃЌDдкХЈСђЫсМгШШЬѕМўЯТгыввДМЗЂЩњѕЅЛЏЗДгІЩњГЩEЃЌDжаКЌга2ИієШЛљЃЌЙЪBЮЊBrCH2C(CH3)=CHCH2BrЃЌCЮЊHOCH2C(CH3)=CHCH2OHЃЌDЮЊHOOCC(CH3)=CHCOOHЃЌEЮЊCH3CH2OOCC(CH3)=CHCOOCH2CH3ЃЎИљОнвбжЊаХЯЂПЩжЊЃЌFЕФНсЙЙМђЪНЮЊ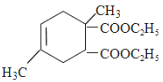 Лђ
Лђ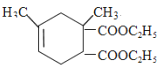 ЃЌдђGЕФНсЙЙМђЪН
ЃЌдђGЕФНсЙЙМђЪН Лђ
Лђ ЁЃ
ЁЃ
ЂйИљОнвдЩЯЗжЮіЃЌBдкЧтбѕЛЏФЦЕФЫЎШмвКжаЗЂЩњЫЎНтЗДгІЩњГЩCЃЌFгыBr2ЗЂЩњМгГЩЗДгІЩњГЩGЃЌЙЪД№АИЮЊЃКШЁДњЗДгІЃЛМгГЩЗДгІЃЛ
ЂкИљОнвдЩЯЗжЮіЃЌAЮЊ![]() ЃЌFЮЊ
ЃЌFЮЊ Лђ
Лђ ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК![]() ЃЌ
ЃЌ Лђ
Лђ ЃЛ
ЃЛ
ЂлBдкЧтбѕЛЏФЦЕФЫЎШмвКжаЗЂЩњЫЎНтЗДгІЩњГЩCЃЌдђBЁњCЗНГЬЪНЮЊЃКBrCH2-C(CH3)=CH-CH2Br+2NaOH![]() HOCH2-C(CH3)=CH-CH2OH+2NaBrЃЛИљОнDКЭEЕФЯрЖдЗжзгжЪСППЩХаЖЯЃЌDдкХЈСђЫсМгШШЬѕМўЯТгыввДМЗЂЩњѕЅЛЏЗДгІЩњГЩEЃЌдђDЁњEЕФЗНГЬЪНЮЊЃКHOOC-C(CH3)=CH-COOH+2C2H5OH
HOCH2-C(CH3)=CH-CH2OH+2NaBrЃЛИљОнDКЭEЕФЯрЖдЗжзгжЪСППЩХаЖЯЃЌDдкХЈСђЫсМгШШЬѕМўЯТгыввДМЗЂЩњѕЅЛЏЗДгІЩњГЩEЃЌдђDЁњEЕФЗНГЬЪНЮЊЃКHOOC-C(CH3)=CH-COOH+2C2H5OH![]() C2H5OOC-C(CH3)=CH-COOC2H5+2H2OЃЌЙЪД№АИЮЊЃКBrCH2-C(CH3)=CH-CH2Br+2NaOH
C2H5OOC-C(CH3)=CH-COOC2H5+2H2OЃЌЙЪД№АИЮЊЃКBrCH2-C(CH3)=CH-CH2Br+2NaOH![]() HOCH2-C(CH3)=CH-CH2OH+2NaBrЃЛHOOC-C(CH3)=CH-COOH+2C2H5OH
HOCH2-C(CH3)=CH-CH2OH+2NaBrЃЛHOOC-C(CH3)=CH-COOH+2C2H5OH![]() C2H5OOC-C(CH3)=CH-COOC2H5+2H2OЃЛ
C2H5OOC-C(CH3)=CH-COOC2H5+2H2OЃЛ
ЂмG гыЧтбѕЛЏФЦШмвКЗДгІЕФЗНГЬЪНЮЊ ЃЌЙЪД№АИЮЊЃК
ЃЌЙЪД№АИЮЊЃК ЁЃ
ЁЃ


 аЁбЇНЬВФЭъШЋНтЖСЯЕСаД№АИ
аЁбЇНЬВФЭъШЋНтЖСЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊQКЭRЕФФІЖћжЪСПжЎБШЪЧ9ЃК22ЃЌдкЗДгІX+2Y=2Q+RжаЃЌЕБ1ЃЎ6gXгыYЭъШЋЗДгІКѓЃЌЩњГЩ4ЃЎ4gRЃЌдђВЮгыЗДгІЕФYКЭЩњГЩЮяQЕФжЪСПжЎБШЮЊ
AЃЎ46:9
BЃЎ32:9
CЃЎ23:9
DЃЎ16:9
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМзЭщжаЕФЬМдзгЪЧsp3дгЛЏЃЌЯТСагУ*БэЪОЬМдзгЕФдгЛЏКЭМзЭщжаЕФЬМдзгдгЛЏзДЬЌвЛжТЕФЪЧ ЃЈ ЃЉ
A. CH3*CH2CH3 B. *CH2ЃНCHCH3 C. CH2ЃН*CHCH2CH3 D. HCЁд*CCH3
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪЕбщЪвРяЭЈГЃгУMnO2гыХЈбЮЫсЗДгІжЦШЁТШЦјЃЌЦфЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃКMnO2 + 4HCl(ХЈ) ![]() MnCl2 + Cl2Ёќ+ 2H2O
MnCl2 + Cl2Ёќ+ 2H2O
ЃЈ1ЃЉгУЕЅЯпЧХЗЈБэЪОИУЗДгІЕчзгзЊвЦЕФЗНЯђКЭЪ§ФПЃК___________ЁЃ
ЃЈ2ЃЉдкИУЗДгІжаЃЌШчга1 mol Cl2ЩњГЩЃЌБЛбѕЛЏЕФHClЕФЮяжЪЕФСПЪЧ___________ЃЌзЊвЦЕчзгЕФЪ§ФПЪЧ_____________ЁЃ
ЃЈ3ЃЉФГЮТЖШЯТЃЌНЋCl2ЭЈШыNaOHШмвКжаЃЌЗДгІЕУЕНКЌгаClO-гыClO3-ЮяжЪЕФСПжЎБШЮЊ1ЁУ1ЕФЛьКЯвКЃЌЗДгІЕФЛЏбЇЗНГЬЪНЪЧ _________________________ ЁЃ
ЃЈ4ЃЉБЈжНБЈЕРСЫЖрЦ№ЮРЩњМфЧхЯДЪБЃЌвђЛьКЯЪЙгУЁАНрВоСщЁБЃЈжївЊГЩЗжЪЧбЮЫсЃЉгыЁА84ЯћЖОвКЁБЃЈжївЊГЩЗжЪЧNaClOЃЉЗЂЩњТШЦјжаЖОЕФЪТМўЁЃЪдИљОнФуЕФЛЏбЇжЊЪЖЗжЮіЃЌдвђЪЧЃЈгУРызгЗНГЬЪНБэЪОЃЉ_________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЮяжЪгыЫсЕФЗДгІжаЃЌжЛБэЯжГіЫсадзїгУЕФЪЧЃЈЁЁЁЁЃЉ
A.CuЃЋ2H2SO4ЃЈХЈЃЉ===CuSO4ЃЋSO2ЁќЃЋ2H2O
B.CЃЋ4HNO3ЃЈХЈЃЉ===CO2ЁќЃЋ4NO2ЁќЃЋ2H2O
C.3FeЃЋ8HNO3ЃЈЯЁЃЉ===3FeЃЈNO3ЃЉ2ЃЋ2NOЁќЃЋ4H2O
D.CuOЃЋ2HNO3ЃЈЯЁЃЉ===CuЃЈNO3ЃЉ2ЃЋH2O
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЮяжЪЕФЗжРрЗНЗЈгаЖржжЗНЗЈЃЌЯТСаЖдЮоЛњЛЏКЯЮяЗжРрШчЭМЃК
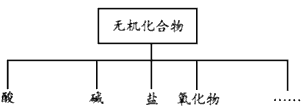
ЃЈ1ЃЉЩЯЭМЫљЪОЕФЮяжЪЗжРрЗНЗЈУћГЦЪЧ____________________ЁЃ
ЃЈ2ЃЉвдKЁЂNaЁЂHЁЂOЁЂSЁЂNжаШЮСНжжЛђШ§жждЊЫизщГЩКЯЪЪЕФЮяжЪЃЌЗжБ№ЬюдкЯТБэжаЂкЁЂЂмЁЂЂоКѓУцЁЃ
ЮяжЪРрБ№ | Ыс | Мю | бЮ | бѕЛЏЮя | ЧтЛЏЮя |
ЛЏбЇЪН | ЂйHNO3 Ђк_______ | ЂлNaOH Ђм_______ | ЂнNa2SO4 Ђо_______ | ЂпCO2 ЂрSO3 | ЂсNH3 |
ЃЈ3ЃЉаДГіЂпгыЩйСПЕФЂлШмвКЗДгІЕФРызгЗНГЬЪН_______________________ЁЃ
ЃЈ4ЃЉаДГіТСгыЂлШмвКЗДгІЕФЛЏбЇЗНГЬЪН______________________ЁЃ
ЃЈ5ЃЉгвЭМЪЧФГбЇаЃЪЕбщЪвДгЛЏбЇЪдМСЩЬЕъТђЛиЕФХЈСђЫсЪдМСБъЧЉЩЯЕФВПЗжФкШнЁЃЯжгУИУХЈСђЫсХфжЦ480 mL 1 molЁЄ LЃ1ЕФЯЁСђЫсЁЃ
ПЩЙЉбЁгУЕФвЧЦїгаЃКЂйНКЭЗЕЮЙмЂкЩеЦПЂлЩеБЂмВЃСЇАєЂнвЉГзЂоСПЭВЂпЭаХЬЬьЦНЁЃ

ЧыЛиД№ЯТСаЮЪЬтЃК
aЃЎИУСђЫсЕФЮяжЪЕФСПХЈЖШЮЊ __________ molЁЄ LЃ1ЁЃ
bЃЎХфжЦЯЁСђЫсЪБЃЌЛЙШБЩйЕФвЧЦїга ______________ (аДвЧЦїУћГЦ)ЁЃ
cЃЎОМЦЫуЃЌХфжЦ480mL 1molЁЄ LЃ1ЕФЯЁСђЫсашвЊгУСПЭВСПШЁЩЯЪіХЈСђЫсЕФЬхЛ§ЮЊ_________mLЁЃ
dЃЎЖдЫљХфжЦЕФЯЁСђЫсНјааВтЖЈЃЌЗЂЯжЦфХЈЖШДѓгк1 molЁЄ LЃ1ЃЌХфжЦЙ§ГЬжаЯТСаИїЯюВйзїПЩФмв§Ц№ИУЮѓВюЕФдвђга ___________ЁЃ
AЃЎЖЈШнЪБЃЌИЉЪгШнСПЦППЬЖШЯпНјааЖЈШн ЁЃ
BЃЎНЋЯЁЪЭКѓЕФЯЁСђЫсСЂМДзЊШыШнСПЦПКѓЃЌНєНгзХОЭНјаавдКѓЕФЪЕбщВйзїЁЃ
CЃЎзЊвЦШмвКЪБЃЌВЛЩїгаЩйСПШмвКШїЕНШнСПЦПЭтУцЁЃ
DЃЎШнСПЦПгУеєСѓЫЎЯДЕгКѓЮДИЩдяЃЌКЌгаЩйСПеєСѓЫЎ ЁЃ
EЃЎЖЈШнКѓЃЌАбШнСПЦПЕЙжУвЁдШКѓЗЂЯжвКУцЕЭгкПЬЖШЯпЃЌБуВЙГфМИЕЮЫЎжСПЬЖШДІЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПбаОПЗЂЯжЃЌNOxКЭSO2ЪЧЮэіВЕФжївЊГЩЗжЁЃ
ЃЈвЛЃЉNOxжївЊРДдДгкЦћГЕЮВЦјЁЃ
вбжЊЃКN2ЃЈgЃЉ+O2ЃЈgЃЉ![]() 2NOЃЈgЃЉЁїHЃН+180.50 kJ/mol
2NOЃЈgЃЉЁїHЃН+180.50 kJ/mol
2COЃЈgЃЉ+O2ЃЈgЃЉ![]() 2CO2ЃЈgЃЉЁїHЃН-566.00kJ/mol
2CO2ЃЈgЃЉЁїHЃН-566.00kJ/mol
ЃЈ1ЃЉЮЊСЫМѕЧсДѓЦјЮлШОЃЌШЫУЧЬсГідкЦћГЕЮВЦјХХЦјЙмПкВЩгУДпЛЏМСНЋNOКЭCOзЊЛЏГЩЮоЮлШОЦјЬхВЮгыДѓЦјбЛЗЁЃаДГіИУЗДгІЕФШШЛЏбЇЗНГЬЪН__________ЁЃ
ЃЈ2ЃЉTЁцЪБЃЌНЋЕШЮяжЪЕФСПЕФNOКЭCOГфШыШнЛ§ЮЊ2LЕФУмБеШнЦїжаЃЌБЃГжЮТЖШКЭЬхЛ§ВЛБфЃЌЗДгІЙ§ГЬЃЈ0-15minЃЉжаNOЕФЮяжЪЕФСПЫцЪБМфБфЛЏШчЭМЫљЪОЁЃ

ЂйTЁцЪБИУЛЏбЇЗДгІЕФЦНКтГЃЪ§K= __________ЃЛШєБЃГжЮТЖШВЛБфЃЌдйЯђШнЦїжаГфШыCOЁЂN2Иї0.8molЃЌЦНКтНЋ____________вЦЖЏЁЃЃЈЬюЁАЯђзѓЁБЁЂЁАЯђгвЁБЛђЁАВЛЁБЃЉ
ЂкЭМжаaЁЂbЗжБ№БэЪОдквЛЖЈЮТЖШЯТЃЌЪЙгУжЪСПЯрЭЌЕЋБэУцЛ§ВЛЭЌЕФДпЛЏМСЪБЃЌДяЕНЦНКтЙ§ГЬжаnЃЈNOЃЉЕФБфЛЏЧњЯпЃЌЦфжаБэЪОДпЛЏМСБэУцЛ§НЯДѓЕФЧњЯпЪЧ__________ЁЃЃЈЬюЁАaЁБЛђЁАbЁБЃЉ
Ђл15minЪБЃЌШєИФБфЭтНчЗДгІЬѕМўЃЌЕМжТnЃЈNOЃЉЗЂЩњШчЭМЫљЪОЕФБфЛЏЃЌдђИФБфЕФЬѕМўПЩФмЪЧ________ЁЃ
ЃЈЖўЃЉSO2жївЊРДдДгкУКЕФШМЩеЁЃШМУКбЬЦјЕФЭбСђМѕХХЪЧМѕЩйДѓЦјжаКЌСђЛЏКЯЮяЮлШОЕФЙиМќЁЃ
ЃЈ3ЃЉгУДПМюШмвКЮќЪеSO2ПЩНЋЦфзЊЛЏЮЊHSO3-ЁЃИУЗДгІЕФРызгЗНГЬЪНЪЧ_____________ЁЃ
ЃЈ4ЃЉШчЭМЕчНтзАжУПЩНЋЮэіВжаЕФNOЁЂSO2ЗжБ№зЊЛЏЮЊNH4+КЭSO42-ЁЃ

ЂйаДГіЮяжЪAЕФЛЏбЇЪН_____________ЃЌбєМЋЕФЕчМЋЗДгІЪНЪЧ_____________
ЂкИУЕчНтЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_______________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП2016Фъ4дТ22ШеЪРНчЕиЧђШеЕФжїЬтЮЊЁАефЯЇЕиЧђзЪдДЃЌзЊБфЗЂеЙЗНЪНЁЊЁЊЬсИпзЪдДРћгУаЇвцЁБЁЃЯТСаЫЕЗЈВЛЗћКЯИУжїЬтЕФЪЧ( )
A. РћгУХЉзїЮяНеИбжЦШЁввДМ
B. ЛиЪеЕиЙЕгЭЃЌжЦБИЩњЮяВёгЭ
C. ЗйЩеЗЯОЩЫмСЯЃЌЗРжЙАзЩЋЮлШО
D. ПЊЗЂРћгУИїжжаТФмдДЃЌМѕЩйЖдЛЏЪЏШМСЯЕФвРРЕ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГРрЛЏбЇЗДгІПЩгУШчЯТЭЈЪНБэЪОЃКA+BЁњC+D+H2O ЧыАДвЊЧѓЛиД№ЮЪЬтЃК
ЃЈ1ЃЉШєCЕФЯЁШмвКЯдРЖЩЋЃЌDЮЊКьзиЩЋЦјЬхЃЌдђBШмвКЕФУћГЦЪЧ______________ЃЌЗДгІжаУПЩњГЩ1 mol H2OЪБзЊвЦЕФЕчзгЪ§ФПЮЊ_________ЁЃЃЈвдNAБэЪОАЂЗќМгЕТТоГЃЪ§ЕФжЕЃЉ
ЃЈ2ЃЉШєAЮЊЕЅжЪЃЌCЁЂDЖМЪЧФмЪЙГЮЧхЪЏЛвЫЎБфЛызЧЕФЦјЬхЁЃдђBЕФЛЏбЇЪНЮЊ_____________ЃЌAЁЂBдкЛЏбЇЗДгІЗНГЬЪНжаЛЏбЇМЦСПЪ§жЎБШЮЊ__________________ЁЃ
ЃЈ3ЃЉШєAЮЊбѕЛЏЮяЃЌCЁЂDжагавЛжжЪЧГЃМћЕФгаЖОЦјЬхЕЅжЪЁЃИУЗДгІЕФРызгЗНГЬЪНЮЊ______________________ЁЃ
ЃЈ4ЃЉШєCЪЧвЛжжМюадЦјЬхЃЌФмЪЙЪЊШѓЕФКьЩЋЪЏШяЪджНБфРЖЃЌDЪЧвЛжжЙЬЬхПЩзїИЩдяМСЃЈжаадЃЉЃЌдђЪЕбщЪвжЦШЁЦјЬхCЕФЗДгІЕФЛЏбЇЗНГЬЪНЪЧ ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com