
| �� |

| 112L |
| 22.4L/mol |
| 2n |
| 4 |
| x |
| 2 |
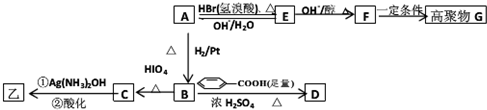
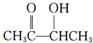 ����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ��
����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ��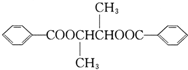 ��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��
��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��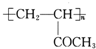 ��
��| 112L |
| 22.4L/mol |
| 2n |
| 4 |
| x |
| 2 |
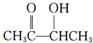 ����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ��
����Ũ���������������������£�CH3CH��OH��CH��OH��CH3�ͱ����ᷢ��������Ӧ����D����D�Ľṹ��ʽΪ�� ��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��
��A�������ᷢ��ȡ����Ӧ����E����E�Ľṹ��ʽΪ��CH3COCH��Br��CH3��E���������ƵĴ���Һ������ȥ��Ӧ����F����F�Ľṹ��ʽΪ��CH3COCH=CH2��F��һ�������·����Ӿ۷�Ӧ����G����G�Ľṹ��ʽΪ��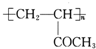 ��
��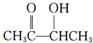 ��G �Ľṹ��ʽΪ
��G �Ľṹ��ʽΪ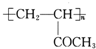 ���ʴ�Ϊ��
���ʴ�Ϊ��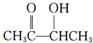 ��
��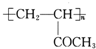 ��
��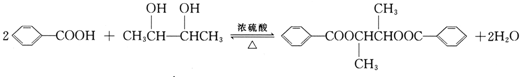 ��
�� ��
��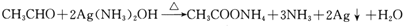 ��
�� ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 3 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
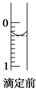 ��֪�������ճ������г��������ᣮ
��֪�������ճ������г��������ᣮ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
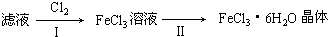
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
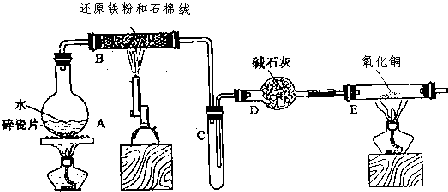
 ��ʵ�����У�����ͼ��ʾװ�ã�β������װ����ȥ����������ʵ�飬������Һ����ε��뵽���У�ʵ������Ԥ�������һ�µ��� ��������
��ʵ�����У�����ͼ��ʾװ�ã�β������װ����ȥ����������ʵ�飬������Һ����ε��뵽���У�ʵ������Ԥ�������һ�µ��� �������� | ѡ�� | ���е����� | ���е����� | Ԥ����е����� |
| A | ��ˮ | �Ȼ�����Һ | ������ɫ���� |
| B | Ũ���� | ͭƬ | �����������壬��Һ���� |
| C | Ũ���� | ��ɰֽ��ĥ�������� | ������������ɫ���� |
| D | ϡ���� | ̼�������������ƵĻ����Һ | ���������������� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
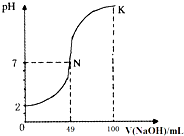 �����£���100mL0.1mol?L-1H2A����Ԫ�ᣩ��Һ����μ���0.2mol?L-1NaOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ��������й�˵����ȷ���ǣ�������
�����£���100mL0.1mol?L-1H2A����Ԫ�ᣩ��Һ����μ���0.2mol?L-1NaOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ��������й�˵����ȷ���ǣ�������| A��H2AΪ��Ԫǿ�� |
| B��K��ʱ��ˮϡ����Һ��c��H+������ |
| C��N���Ӧ��Һ�У�c��Na+��=c��A2-��+c��HA-�� |
| D��K���Ӧ��Һ������Ũ���ɴ�С��˳��Ϊ��c��A2-����c��Na+����c��OH-����c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
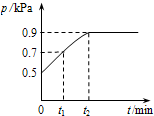 ��1.0L�ܱ������з���0.10 mol X����һ���¶��·�����Ӧ��X��g��?Y��g��+Z��g����H��0������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������
��1.0L�ܱ������з���0.10 mol X����һ���¶��·�����Ӧ��X��g��?Y��g��+Z��g����H��0������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������A���ӷ�Ӧ��ʼ��t1ʱ��ƽ����Ӧ����v��X��=
| ||
| B�����¶��´˷�Ӧ��ƽ�ⳣ��K=0.32 | ||
| C�������ƽ����ϵ��Y�ĺ�������������ϵ�¶Ȼ����Z���� | ||
| D�������������䣬�ٳ���0.1 mol ����X��ƽ�������ƶ���X��ת�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
 Ϊ̼ԭ�ӣ�
Ϊ̼ԭ�ӣ� Ϊ��ԭ�ӣ���ÿ��̼ԭ����Χ�����������Ĺ�ԭ����
Ϊ��ԭ�ӣ���ÿ��̼ԭ����Χ�����������Ĺ�ԭ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ۢ� | B���٢� | C���٢ۢ� | D���٢� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com