
 50mL 0.55mol/L������50mL 0.50mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
50mL 0.55mol/L������50mL 0.50mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ��������
��2������Ӳֽ�壬����һ��������ɢʧ��
��3��Ũ����ϡ��ʱ�ų�������
��� �⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β����������
�ʴ�Ϊ�����β����������
��2�����ձ����粻��Ӳֽ�壬��ʹһ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
��3��Ũ����ϡ��ʱ�ų�����������50gŨ�������������з�Ӧ��������ʵ����ȣ����ų�������ƫ���¶Ȳ�ƫ���к�����ֵ����ȣ�
�ʴ�Ϊ������ȣ�
���� ���⿼��ѧ���й��к��ȵIJⶨ֪ʶ�����Ը�����ѧ֪ʶ���лش�ע����к��ȸ�������⣬�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �о���Ա������һ�֡�ˮ������أ����ܷ�ӦΪ��5Mn02+2Ag+2NaCl=Na2Mn5O10+2AgCl����ͼ�á�ˮ�����Ϊ��Դ���NaCl��Һ��ʵ���У�X�缫������ɫ�����ݳ��������йط�����ȷ���ǣ�������
�о���Ա������һ�֡�ˮ������أ����ܷ�ӦΪ��5Mn02+2Ag+2NaCl=Na2Mn5O10+2AgCl����ͼ�á�ˮ�����Ϊ��Դ���NaCl��Һ��ʵ���У�X�缫������ɫ�����ݳ��������йط�����ȷ���ǣ�������| A�� | IΪ��������缫��ӦʽΪAg+Cl--e-=AgCl | |
| B�� | ��ˮ�������Na+�������������ƶ� | |
| C�� | ÿת��1mole-��U��������0.5mol H2O | |
| D�� | ��ʼʱU����Y������pH������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
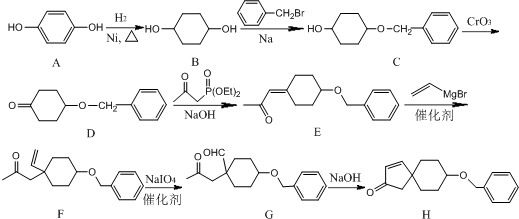
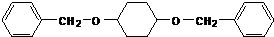 ����C��D�ķ�Ӧ������������Ӧ��
����C��D�ķ�Ӧ������������Ӧ��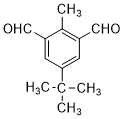 ��
��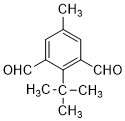 ��
�� Ϊ�л�ԭ���Ʊ�
Ϊ�л�ԭ���Ʊ� �ĺϳ�·������ͼ�����Լ���ѡ����ѡ���ʵ��л��ܼ������ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ����ѡ���ʵ��л��ܼ������ϳ�·������ͼʾ�����£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�| �¶� ʵ����� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | 3.4�� |
| 2 | 27.0 | 27.4 | 27.2 | 32.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 4 | 26.4 | 26.2 | 26.3 | 29.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com