
����Ŀ����1��ȡ20 mL pH��3��CH3COOH��Һ������0.2 mol��L��1�İ�ˮ�������Һ�����Ա仯��ͼ������백ˮǰCH3COOH�ĵ����(���ӵ���İٷ���)Ϊ______________������0��10 mL�İ�ˮ����������ǿ��ԭ��_________________________��

��2��������粒�������ˮ�����0.1 mol��L��1��Һ����֪����ĵ���ƽ�ⳣ��ΪKa��һˮ�ϰ��ĵ���ƽ�ⳣ��ΪKb��ʵ�鷢�����߽�����ȣ���д�������ˮ������ӷ���ʽ______________________��ˮ��ƽ�ⳣ���ı���ʽ____________________��
���𰸡�1% �����кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ NH4+��CH3COO����H2O![]() NH3��H2O��CH3COOH K��
NH3��H2O��CH3COOH K��![]()
��������
(1)����ͼ�����������ˮ�����Ϊ10mLʱ��������ǿ��˵���պ��������ȫ��Ӧ�����Դ����Ũ��Ϊ��![]() =0.1molL-1���ٸ���pH=3��CH3COOH��Һ����������Ũ��Ϊ10-3molL-1������CH3COOH�ĵ����Ϊ
=0.1molL-1���ٸ���pH=3��CH3COOH��Һ����������Ũ��Ϊ10-3molL-1������CH3COOH�ĵ����Ϊ ![]() ��100%=1%�������кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ����˼���0��10 mL�İ�ˮ������������ǿ���ʴ�Ϊ��1%�������кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ��
��100%=1%�������кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ����˼���0��10 mL�İ�ˮ������������ǿ���ʴ�Ϊ��1%�������кͷ�Ӧ���У���Һ������(笠����ӣ����������)Ũ�����������ﵽ��ѧ������ʱ����Ũ�ȴ����ֵ��
(2)����������������Σ�������ǿ����ʣ�����ˮ����ȫ���룬笠����Ӻ���������Ӷ�ˮ�⣬��ѧ����ʽΪCH3COONH4+H2OCH3COOH+NH3H2O�����ӷ���ʽΪ��CH3COO-+NH4++H2OCH3COOH+NH3H2O��ˮ��ƽ�ⳣ���ı���ʽK=![]() =
=![]() =
=![]() =
=![]() ���ʴ�Ϊ�� CH3COO-+NH4++H2OCH3COOH+NH3H2O��K=
���ʴ�Ϊ�� CH3COO-+NH4++H2OCH3COOH+NH3H2O��K=![]() ��
��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ��ɱ���ܱ������д������·�Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H<0�����з����в���ȷ����( )
CO2(g)��H2(g) ��H<0�����з����в���ȷ����( )

A. ͼ���о�����t0ʱ�����¶ȶԷ�Ӧ���ʵ�Ӱ��
B. ͼ���о�����t0ʱ����ѹǿ(��С�ݻ�)��ʹ�ô����Է�Ӧ���ʵ�Ӱ��
C. ͼ���о����Ǵ����Ի�ѧƽ���Ӱ�죬�Ҽ�ʹ���˴���
D. ͼ���о������¶ȶԻ�ѧƽ���Ӱ�죬���ҵ��¶Ƚϸ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ʴ�йص�˵������ȷ����
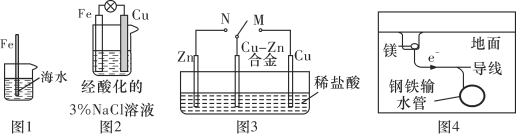
A. ͼ1�У����뺣ˮ�е�������Խ�����˸�ʴԽ����
B. ͼ2�У����ձ��еμӼ���KSCN��Һ����Һ��Ѫ��ɫ
C. ͼ3�У�������M������Nʱ��Cu��Zn�Ͻ�ĸ�ʴ��������
D. ͼ4�У�������������������������������ֹ���¸����ܵ��ĸ�ʴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й�������Լ���ƴ������ʱ����������¯��ʯ�㻯�ʯ(��ʯ��)�ļ��գ���¯��ʯ(ZnCO3)����ͭ��(��Ҫ�ɷ�Cu2O��ľ̿�ۻ�ϼ�����800�����ҿ��Ƶ���ƽ����Ƶ���ʯ�𡣻ش��������⣺
(1)пԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽΪ___________��ͭԪ�ػ�̬ԭ���е�δ�ɶԵ�����Ϊ___________��
(2)����п���ڹ�����ˮ�γ�[Zn(NH3)]SO4��Һ��
��[Zn(NH3)4]SO4�У������ӵ����幹����___________�� [Zn(NH3)4]2+�к��еĻ�ѧ����_______��
��NH3�����У�����ԭ�ӵĹ���ӻ�����Ϊ___________�� NH3��H2O�е��ܽ��___________(������������С��)��ԭ����_________________________________��
(3)ͭ�ĵ�һ������ΪI1(Cu)=745.5 kJ��mol��1���ڶ�������Ϊl2(Cu)=1957.9 kJ��mol��1��п�ĵ�һ������ΪI1(Zn)=906.4 kJ��mol��1���ڶ�������ΪI2(Zn)=1733.3kJ��mo1��1��I2(Cu)>I2(Zn)��ԭ����_______________

(4)Cu2O����ľ����ṹ��ͼ��ʾ��O2������λ��Ϊ___________����Cu2O���ܶ�Ϊdg��cm��3��������a=___________nm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO2��һ�����۵�̼��Դ�����ۺ����þ�����Ҫ���塣�ش��������⣺
��1��CO2���Ա�NaOH��Һ�������£���������ҺpH��13��CO2��Ҫת��Ϊ________(д���ӷ���)����������Һc(HCO3-)��c(CO32-)��2��1����ҺpH��__________��(�����£�H2CO3��K1��4��10��7��K2��5��10��11)
��2����֪25�棬NH3��H2O��Kb��1.8��10��5��H2SO3��Ka1��1.3��10��2��Ka2��6.2��10��8������ˮ��Ũ��Ϊ2.0 mol��L��1����Һ�е�c(OH��)��________________mol��L��1����SO2ͨ��ð�ˮ�У���c(OH��)����1.0��10��7mol��L��1ʱ����Һ�е�c(SO32-)/c(HSO3-)��__________________��
��3���ڻ�ѧ�����в���K2CrO4Ϊָʾ������AgNO3����Һ�ζ���Һ��Cl��������Ag����CrO42-����ש��ɫ������ָʾ����ζ��յ㡣����Һ��Cl��ǡ�ó�����ȫ(Ũ�ȵ���1.0��10��5mol��L��1)ʱ����Һ��c(Ag��)Ϊ________mol��L��1����ʱ��Һ��c(CrO42-)����__________mol��L��1��(��֪Ag2CrO4��AgCl��Ksp�ֱ�Ϊ2.0��10��12��2.0��10��10)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���⻯ﮣ�LiH���ڸ���Ŀ��������ȶ����ڣ���ˮ�����ܹ�����ȼ�ա�ij�С����ʹ������װ���Ʊ�LiH���塣
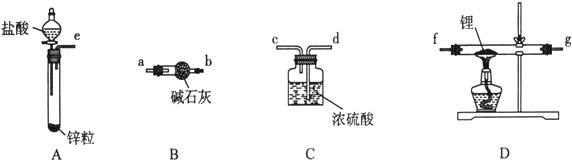
��ͬѧ��ʵ�鷽�����£�
��1����������װ���ӣ���������װ�ð���������������˳��Ϊ________________������ҩƷǰ����Ҫ���е�ʵ�������____________������д������IJ���������������װ��B��������___________��
��2������ҩƷ�������Ӵ��Լ�ƿ��ȡ��һ��������ﮣ�����ʯ���ܷ⣩��Ȼ���ڼױ��н�ϴ���Σ��ò�����Ŀ����____________________��Ȼ����ٰ�﮷��뵽ʯӢ���С�
��3��ͨ��һ��ʱ�����������ʯӢ�ܣ�ͨ������������___________________________���ڼ���D����ʯӢ��֮ǰ��������е�ʵ�������__________��
��4������һ��ʱ���ֹͣ���ȣ�����ͨ������ȴ��Ȼ��ȡ��LiH��װ�뵪���ƿ������ڰ�������ȡ����������Ŀ����Ϊ�˱���LiH������е�ˮ�����Ӵ�������Σ�գ���Ӧ����ʽΪ_____________��
��5��ȷ�����ƵõIJ�Ʒ0.174g����һ��������������ˮ��Ӧ���ռ�������470.4 mL���ѻ���ɱ�״���������Ʒ��LiH��Li�����ʵ���֮��Ϊ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ij�ᾧˮ����Ļ�ѧʽΪAnH2O��A����Է�������ΪM������ag�û�����������ᾧˮȫ��ʧȥ��ʣ��IJ�������Ϊbg����n=_______
��2��ijҺ̬������X2Y4�����������ȼ�ϡ�16gX2Y4��һ������O2��ǡ����ȫȼ�գ���Ӧ����ʽX2Y4(l)+O2(g)=X2(g)+2Y2O(l)������ȴ����������X2�ڱ�״�����ܶ�Ϊ1.25g/L����
��X2��Ħ������Ϊ_________
��Y2O �������Һ̬�����X2Y4�Ļ�ѧʽΪ__________
�۷�ӦǰO2���������״���£�Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ȵ�ԭ��������17����ô��Ԫ����Ԫ�����ڱ��е�λ���� �� ��
A.��2���ڢ�A��B.��3���ڢ�A��
C.��3���ڢ�A��D.��3���ڢ�B��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ԫ�����ڱ���˵������ȷ���ǣ� ��
A. Ԫ�����ڱ���7�����ڡ�18����B. 38��Ԫ��λ�ڵ������ڵ�IIA��
C. �ǽ���Ԫ�ؾ�λ�����ڱ��Ҳ�D. �������ڹ���18��Ԫ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com