
Na��Cu��O��Si��S��Cl�dz���������Ԫ�ء�
(1)Naλ��Ԫ�����ڱ���________���ڵ�________�壻S�Ļ�̬ԭ�Ӻ�����________��δ�ɶԵ��ӣ�Si�Ļ�̬ԭ�Ӻ�������Ų�ʽΪ________________________��
(2)�á�>����<����գ�
| ��һ������ | ���Ӱ뾶 | �۵� | ���� |
| Si____S | O2��____Na�� | NaCl____Si | H2SO4____HClO4 |
(3)CuCl(s)��O2��Ӧ����CuCl2(s)��һ�ֺ�ɫ���塣��25 �桢101 kPa�£���֪�÷�Ӧÿ����1 mol CuCl(s)������44.4 kJ���÷�Ӧ���Ȼ�ѧ����ʽ��________________________________________________________________________
________________________________________________________________________��
(4)ClO2������ˮ�ľ�������ҵ�Ͽ���Cl2����NaClO2��Һ��ȡClO2��д���÷�Ӧ�����ӷ���ʽ�����������ת�Ƶķ������Ŀ��________________________________________________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��.�����й�˵������ȷ����___ _____��
_____��
A����ͬ���͵����Ӿ��壬������Խ���γɵľ���Խ�ȶ�
B��NH3��H3O+�ǵȵ����壬��˽ṹ����������
C�����ǻ�����ȩ�е���ڶ��ǻ�����ȩ��ԭ����ǰ�ߴ��ڷ�����������ߴ���
���Ӽ����
D��H3O����HF2����[Ag(NH3)2]���о�������λ��
��.̼���仯��������Ȼ���й㷺���ڡ�
(1)��̬̼ԭ�ӵļ۵����Ų�ͼ�ɱ�ʾΪ ������������������ͬ
δ�ɶԵ������Ĺ��ɽ����� ����Ԫ�ط��ţ�
(2)��һ�����ܣ�C��N��O��F����Ԫ���ɴ�С˳��___ _ ��
ԭ���� ��
HCN��NF3���ӹ��ͷֱ��� ��
(3)��������ˮ���ӵĿռ����з�ʽ����ʯ�������ơ�ÿ��������ƽ��ռ��________��ˮ���ӣ�����������ʯ�������з�ʽ��ͬ��ԭ����__________________________��
(4)C60�ľ����У�����Ϊ���������ѻ�����֪������C60�������ļ����̾���Ϊ
d cm���ɼ���C60������ܶ�Ϊ________g/cm3��
(5)��д��һ����Ӧ����ʽ�Ա������Ӧǰ̼ԭ�ӵ��ӻ���ʽΪsp2����Ӧ���Ϊsp3��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ���в���Ҫ�õ����������� �� ��
A������0.1mol/Lʳ����Һ500mL B������ C���ܽ� D����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ������Ҫ0.1 mol/L������920 mL��ijͬѧ�����ܶ�Ϊ1.84 g/cm3�����ʵ���������Ϊ98%��Ũ����������ơ��Իش�
��1����ѡ��_________����ƿ������ţ���
A��50 mL B��100 mL C��250 mL D��1000 mL
��2������ȡ98%Ũ��������Ϊ__________mL��
��3�����й�������ƿ��ʹ�÷����У���ȷ����________������ţ���
A.ʹ��ǰҪ�����Ƿ�©ˮ B.������ƿ��ֱ���ܽ�����ϡ��Һ��
C.��Һδ����ȴ��ע������ƿ�� D.������ƿ��ת����Һʱ���ò���������
��4������Ũ��ƫ�͵�ԭ�������________������ţ���
A������ƿ��ԭ������������ˮ B����Һʱ��������Һ������ƿ��
C������ƿʢ��������Һ��ʹ��ǰδϴ�� D������ʱ���ӿ̶��ߺ�Һ��
E��δϴ���ձ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
[��ѧ�����ʽṹ������]ʯīϩ[��ͼ(a)��ʾ]��һ���ɵ���̼ԭ�ӹ��ɵ�ƽ��ṹ����̼���ϣ�ʯīϩ�в���̼ԭ�ӱ���������ƽ��ṹ�ᷢ���ı䣬ת��Ϊ����ʯīϩ[��ͼ(b)��ʾ]��
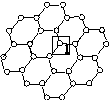 ��
��
(a)ʯīϩ�ṹ����������(b)����ʯīϩ�ṹ
(1)ͼ(a)�У�1��C������C�γɦҼ��ĸ���Ϊ________��
(2)ͼ(b)�У�1��C���ӻ���ʽ��________����C������C�γɵļ���________(�>����<������)ͼ(a)��1��C������C�γɵļ��ǡ�
(3)����ͼ(b)��ʾ������ʯīϩ��ɢ��H2O�У�������ʯīϩ�п���H2O�γ������ԭ����________(��Ԫ�ط���)��
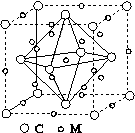
(4)ʯīϩ��ת��Ϊ����ϩ(C60)��ij����M��C60���Ʊ�һ�ֵ��³������ϣ�������ͼ��ʾ��Mԭ��λ�ھ������������ڲ����þ�����Mԭ�ӵĸ���Ϊ________���ò��ϵĻ�ѧʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
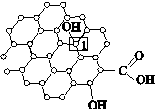
A.[���ʽṹ������]
����NaOH��Cu(OH)2����Һ�����ڼ���ȩ����Ҳ�����ں������Ƿ�Ӧ�Ʊ�����Cu2O��
(1)Cu����̬��������Ų�ʽΪ____________________��
(2)��OH����Ϊ�ȵ������һ�ַ���Ϊ______________(�ѧʽ)��
(3)ȩ����̼ԭ�ӵĹ���ӻ�������________��1 mol��ȩ�����к��еĦҼ�����ĿΪ________��
(4)����NaOH��Cu(OH)2����Һ����ȩ��Ӧ�Ļ�ѧ����ʽΪ__________________________��
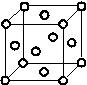
(5)Cu2O��ϡ����������Cu��CuSO4��ͭ�����ṹ��ͼ��ʾ��ͭ������ÿ��ͭԭ����Χ���������ͭԭ����ĿΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �ҹ���ѧ�����Ƴ�һ�д��������������¸�Ч�������м�ȩ���������䷴Ӧ���£�HCHO+O2 ���� CO2+H2O�������й�˵����ȷ����
�ҹ���ѧ�����Ƴ�һ�д��������������¸�Ч�������м�ȩ���������䷴Ӧ���£�HCHO+O2 ���� CO2+H2O�������й�˵����ȷ����
A���÷�ӦΪ���ȷ�Ӧ B��CO2�����еĻ�ѧ��Ϊ�Ǽ��Լ�
C��HCHO�����мȺ������ֺ��м� D��ÿ����1.8gH2O����2.24L O2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ��X��Y��Z��W��Q��Ԫ�����ڱ��е����λ����ͼ1��ʾ������˵����ȷ����
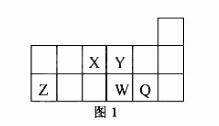
A.Ԫ��X��Ԫ��Z����������ϼ�֮�͵���ֵ����8
B.ԭ�Ӱ뾶�Ĵ�С˳��Ϊ��rX��rY��rZ��rW��rQ
C.����Y2���� Z 3���ĺ���������͵��Ӳ���������ͬ
Z 3���ĺ���������͵��Ӳ���������ͬ
D.Ԫ��W������������Ӧ��ˮ��������Ա�Q��ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧʽ���ܱ�ʾ���ʵ���ɣ����ܱ�ʾ���ʵ�һ�����ӵ��ǣ� ��
A.NaOH B.SiO2 C.Fe D.C3H8
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com