
����Ŀ����ԭ�������ĵ��������ֶ�����Ԫ��(����ĸx�ȱ�ʾ)ԭ�Ӱ뾶����Դ�С��������ۻ�����۵ı仯����ͼ��ʾ��
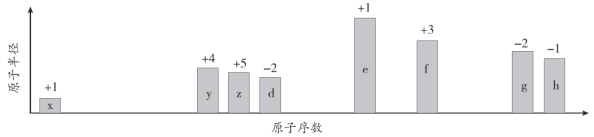
�����жϳ���Ԫ�ػش����⣺
��1��f��Ԫ�����ڱ���λ����__________________��
��2���Ƚ�d��e�������ӵİ뾶��С(�û�ѧʽ��ʾ����ͬ)______��_______���Ƚ�g��h������������Ӧ��ˮ���������ǿ���ǣ�_______��____��
��3������x2d2�ĵ���ʽ��____________________��
��4����֪1mol e�ĵ���������d2��ȼ�գ��ָ������£��ų�255.5kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��____________________________________________��
��5��д���ö��Ե缫���eh��Һ�Ļ�ѧ����ʽ��________________________________��
���𰸡��������ڢ� A�� r(O2-) r(Na+) HClO4 H2SO4 ![]() 2Na(s)+O2(g)=Na2O2(s) ��H= -511kJ��mol-1
2Na(s)+O2(g)=Na2O2(s) ��H= -511kJ��mol-1 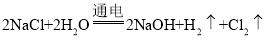
��������
ͬ���ڴ�������ԭ�Ӱ뾶���μ�С��ͬ������ϵ���ԭ�Ӱ뾶������������Ԫ�ص�������۵���������������۵ľ���ֵ=8���������������ݴ˷�������xΪH��yΪC��zΪN��dΪO��eΪNa��fΪAl��gΪS��hΪCl���ݴ˷�����
����������ۻ�����ۣ��Լ�ԭ��������ԭ�Ӱ뾶���Ƴ�xΪH��yΪC��zΪN��dΪO��eΪNa��fΪAl��gΪS��hΪCl��
��1��fΪAl��λ�ڵ������ڵڢ� A�壻
��2��d��e��������O2-��Na+�����Ǻ�������Ų���ͬ��ԭ������Խ�뾶ԽС����r(O2-)>r(Na+)���ǽ�����Խǿ��������������Ӧˮ���������Խǿ��gΪS��hΪCl��Cl�ķǽ�����ǿ��S����HClO4������ǿ��H2SO4��
��3��x2d2�Ļ�ѧʽΪH2O2����ṹʽΪH-O-O-H������ʽΪ![]() ��
��
��4��Na��������ȼ������Na2O2����1mol Na��������ȼ�գ�����Na2O2���Ȼ�ѧ��Ӧ����ʽΪNa(s)+![]() O2(g)=
O2(g)= ![]() Na2O2(s) ��H= -255.5kJ��mol-1����2Na(s)+O2(g)=Na2O2(s) ��H= -511kJ��mol-1��
Na2O2(s) ��H= -255.5kJ��mol-1����2Na(s)+O2(g)=Na2O2(s) ��H= -511kJ��mol-1��
��5�����Բ��ϵ�ⱥ��ʳ��ˮ���䷴Ӧ����ʽΪ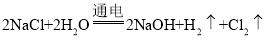 ��
��


 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������������װ��(��)�����÷�����������������һ����ԭ�ӻ��˸��ǻ��γɣ�����˵������ȷ����
A. ������ֻ�밲����װ����˵���÷��Ӳ��Ǵ�
B. �÷���������ԭ��һ������
C. �÷����ܺ�Na�����û���Ӧ����H2
D. �÷��ӱ����ϵ�һ����ԭ�ӱ���C4H9ȡ�����õ�ͬ���칹����12��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������屽�ķϴ�������Ҫ��FeBr3�������塢����Ϊԭ�ϣ���ȡ��ˮFeCl3����ı���Һ��ѡ�õķ����ܴﵽ��Ӧʵ��Ŀ�ĵ���

A. ��װ�âټ����Լ���ȡ����
B. ��װ��������FeBr3��Һ�е�������
C. ��װ�â۷����FeCl3��Һ������ѡ��װ�âܷ���
D. ��װ������FeCl3��Һ�������ɣ��ɵ���ˮFeCl3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������裨Si3N4����һ�������մɲ��ϣ���������ʯӢ�뽹̿�ڸ����µĵ������У�ͨ�����·�Ӧ�Ƶã�3SiO2(s) + 6C(s) +2N2(g)![]() Si3N4(s) +6CO(g)
Si3N4(s) +6CO(g)
(1)�÷�Ӧ��ƽ�ⳣ������ʽΪK=___________________��
(2)��ͬ�¶���SiO2��ƽ��ת������ʱ��ı仯��ͼ��ʾ����÷�ӦΪ___________��Ӧ�������������������������������¶ȣ���ƽ�ⳣ��ֵ��___________�������������� ��С����������������
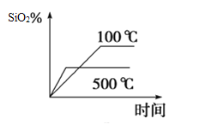
(3)��֪��Ӧ�ﵽƽ��ʱ��CO����������Ϊv (CO) =18 mol��L-1��min-1����N2��������Ϊv(N2)=
____________mol��L-1��min-1��
(4)��Ӧ�ﵽƽ��ʱ��ѹ�������������ƽ�⽫��________��Ӧ�����ƶ�����������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽ����ȷ���ǣ� ��
A��4g��������ȫȼ������SO2���ų�37 kJ������S(s)+O2(g)=SO2(g) ��H= -296kJ/mol
B��1molN2��3molH2��ij�ܱ������з�Ӧ�ų�73kJ��������Ӧ���Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)![]() 2NH3(g) ��H=-73kJ/mol
2NH3(g) ��H=-73kJ/mol
C������ı�ȼ����Ϊ-890.3kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��CH4(g)+2O2(g)��CO2(g)+ 2H2O(g) ��H=-890.3kJ��mol-1
D��ǿ��ǿ����к���Ϊ-57.3 kJ/mol�� Ba(OH)2(aq)+H2SO4(aq)��BaSO4(S)+2H2O(l) ��H=-114.6kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʹ�������Դ��չ����̼����������Ϊ��ѧ���о�����Ҫ���⡣�������״������ʵ����ȼ�ϣ�������ȼ�ϵ�ء�
��1����֪����2CH3OH(l)+3O2(g)=2CO2(g)+4H2O(g) ��H1= -1275.6 kJ��mol-1����2CO(g)+O2(g)=2CO2(g) ��H2=-566.0 kJ��mol-1����H2O(g)=H2O(l) ��H3= -44.0 kJ��mol-1��
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ��_______________��
��2����ҵ��һ��ɲ������·�Ӧ���ϳɼ״���2H2(g)+CO(g)![]() CH3OH(g) ��H= -90.8kJmol-1��
CH3OH(g) ��H= -90.8kJmol-1��
��ij�¶��£���2mol CO��6mol H2����2L���ܱ������У���ַ�Ӧ10min�ﵽƽ��ʱ���c(CO)=0.2mol/L����CO��ת����Ϊ____����CH3OH��ʾ�ù��̵ķ�Ӧ����v(CH3OH)=______��
��Ҫ��߷�Ӧ2H2(g)+CO(g)CH3OH(g)��CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ��_______��
a������ b��������� c������CO��Ũ�� d������H2 e������������� f��������״�
��3����ͼ��һ����ѧ���̵�ʾ��ͼ��

��ͼ�м׳���________װ��(��������������ԭ�����)������OH������________��(����CH3OH������O2��)��
��д��ͨ��CH3OH�ĵ缫�ĵ缫��Ӧʽ�� ____________________________��
���ҳ����ܷ�Ӧ�����ӷ���ʽΪ______________________________________��
�ܵ��ҳ���B(Ag)������������5.40gʱ����ʱ����ij�缫����1.60gij����������е�ij����Һ������________(�����)��
A��MgSO4 B��CuSO4 C��NaCl D��Al(NO3)3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��������������ԭ�����͵���
�ٹ�ҵ�ϳɰ�N2(g)+3H2(g)![]() 2NH3(g) ��H<0����Ӧ����ѡ�����
2NH3(g) ��H<0����Ӧ����ѡ�����
��ʵ���ҿ������ű���ʳ��ˮ�ķ����ռ�����
�����Ṥҵ��2SO2+O2![]() 2SO3������O2��Ũ�����������SO2��ת����
2SO3������O2��Ũ�����������SO2��ת����
�ܶ�CO(g)+NO2(g)![]() CO2(g) + NO(g)ƽ����ϵ����ѹǿ��ʹ��ɫ����
CO2(g) + NO(g)ƽ����ϵ����ѹǿ��ʹ��ɫ����
A. �ڢ� B. �ڢ� C. �٢� D. �٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ | CH3COOH | H2CO3 | HC1O |
����ƽ�ⳣ�� | 1.7��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
��ش��������⣺
��1��CH3COOH��H2CO3��HC1O��������ǿ������˳��Ϊ______________________��
��2��������0.1 mol��L-1��CH3COOH��Һ�������{�¶ȣ�����4�ֱ���ʽ�������������______��
A��c(H+) B��c(H+)/c(CH3COOH) C�� c(H+)��c(OH-) D��c(CH3COO��)��c(H+)/c(CH3COOH)
��3��ȡ0.10mol CH3COOH �������ᣩ��������ʵ�飬����䵼����������ˮ���仯��ͼ��ʾ���Ƚ�a��b���������ʣ��>����<����=������

n(H+)��a_____b��c(CH3COO-)��a_____b����ȫ�к�ʱ����NaOH�����ʵ�����a_____b��
��4��H+Ũ����ͬ�������������ҺA(���ᣩ��B(CH3COOH)�քe��п�۷�Ӧ����������һ����Һ�д���п���ų�������������ͬ��������˵����ȷ����__________����д��ţ�
�ٷ�Ӧ����Ҫ��ʱ��B>A �ڿ�ʼ��Ӧʱ������A>B
�۲μӷ�Ӧ��п�����ʵ���A=B ��A����пʣ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���E��ҽҩ�ϳ��м��塣��ϳ�·�����£�

��1��![]() �Ĺ���������Ϊ_____________��E�ķ���ʽΪ_______________��
�Ĺ���������Ϊ_____________��E�ķ���ʽΪ_______________��
��2��B��D�Ĺ����У�B���ӵ��Ȼ�ȫ��������д���÷�Ӧ����ʽ��_________________��
��3��D��E�ķ�Ӧ�������£�

д����Ӧ��ķ�Ӧ���ͣ���_____________����_____________
��4��д����������������A������ͬ���칹��Ľṹ��ʽ___________________��
a����������������ͭ����Һ��Ӧ����ש��ɫ����
b������������Na2CO3��Ӧ���ͷų�����
��5����д����CH2=CH2Ϊ��Ҫԭ�ϣ����Լ����ã��Ʊ�OHC��CHO���Ҷ�ȩ���ĺϳ�·������ͼ����ע����Ӧ������_______�����ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B����
B����![]() Ŀ����
Ŀ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com