
��ѧʵ�����о��������ʵĻ�����
(1)�����й�ʵ�������������ݺ�������________(�����)��
a��������������CuSO4��5H2O����ⶨ�ᾧˮ��������
b���ø����pH��ֽ�ⶨŨ�����pH
c���ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ
(2)ij��ˮ��Ʒ�к���һ������Na����CO32-��SO32-��ij�о�С�����ⶨ����SO32-��Ũ�ȡ�
ʵ�鷽����
��.���ձ�ʢȡ��ˮ����������������̿����ȥ��ˮ�е����ʣ����ˣ�ȡ��Һ��
��.��ȷ��ȡ20.00 mL���˺��ˮ������ѡ��ʹ����ɫ��0.1 mol/L KMnO4(H2SO4�ữ)��Һ���еζ���
��.��¼���ݣ����㡣
�����еζ���ʽ�У����������(�гֲ��ѷ���ȥ)______(����ĸ���)��
�ڵζ������У��йط�Ӧ�����ӷ���ʽ��__________________________________��
(3)ijͬѧ�Ʊ�Fe(OH)3���壺�ýྻ���ձ�ȡ��������ˮ���������ڣ����ձ��еμ� 1 mol/L��FeCl3��Һ���������ò��������裬�����Һ����ǡ���ͬѧ�Ʊ�����ʧ�ܵ�ԭ������������������������Ϊ�ɹ��Ƶ�Fe(OH)3���������������_________��
(4)����ͼװ�ý���CO2���ʵ��й�ʵ�顣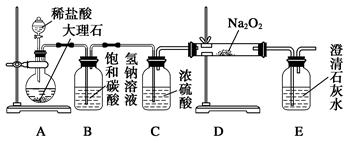
�Լ�ƿB��ʢ�б���NaHCO3��Һ����Ŀ����:
_______________________ __________��
�ڷ�Ӧ�����У�E�г���ʯ��ˮ����ǣ�E�еĻ����ϵ�г����ڵ���ƽ�⡢ˮ��ƽ���⣬�������ܽ�ƽ�⣬�÷���ʽ��ʾ���ܽ�ƽ���ϵ��
____________________ ___________��
(1)c(2)��b����2MnO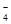 +5SO
+5SO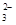 +6H+��2Mn2++5SO
+6H+��2Mn2++5SO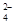 +3H2O
+3H2O
(3)���赼�½���۳���Һ������ĺ��ɫ(�������ɫ)
(4)�ٳ�ȥHCl�������� ��CaCO3(s)  Ca2+(aq)+CO
Ca2+(aq)+CO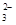 (aq)
(aq)
���������������1��a�����������ܺ�ˮ������Ӧ��ʵ�鲻��������������CuSO4��5H2O����ⶨ�ᾧˮ����������a����ȷ��
b��Ũ���������ˮ�ԣ������ø����pH��ֽ�ⶨŨ�����pH��b����ȷ��
c����Ͳ���Զ�����0.1ml�������ù��Ϊ20 mL����Ͳ����ȡ16.8 mL��Na2CO3��Һ��c��ȷ����ѡc��
��2�������Ը��������ҺӦ�÷�����ʽ�ζ����У�a�����Ը��������Һ���ڼ�ʽ�ζ����У����a����ȷ��b��ȷ��Ӧ���ø��������Һ�ζ���ˮ��ѡ��c����ȷ����ѡb��
�ڸ�����ؾ���ǿ�����ԣ��ܰ�SO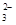 ���������Եζ������У��йط�Ӧ�����ӷ���ʽ��2MnO
���������Եζ������У��йط�Ӧ�����ӷ���ʽ��2MnO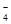 +5SO
+5SO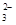 +6H+��2Mn2++5SO
+6H+��2Mn2++5SO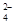 +3H2O��
+3H2O��
��3���������Ȼ���������Һ�����ˮ���Ʊ������������壬����ֱ��Һ���Ϊ���ĺ��ɫֹͣ���ȣ����貣�������衣���Ը�ʵ��ʧ�ܵ�ԭ���ǽ��赼�½���۳���
��4���������ӷ������ɵ�CO2�к����Ȼ������壬���Ա���̼��������Һ�������dz�ȥHCl�������塣
��̼��������������ʣ������ܽ�ƽ�⣬����ʽ��CaCO3(s)  Ca2+(aq)+CO
Ca2+(aq)+CO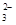 (aq)��
(aq)��
���㣺���黯ѧʵ�����������������ԭ��Ӧ����ʽ����д����������������Ʊ�������ľ������ܽ�ƽ���
��������ѧʵ�鳣��������ʹ�÷����ͻ�ѧʵ����������ǽ��л�ѧʵ��Ļ������Ի�ѧʵ��Ŀ����벻����ѧʵ��Ļ������������Ա����������ڸ߿�������һ�����ǵ������⣬��һ�����dz������ۺ�ʵ�������У���ǰ����˵�������Ȼ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ���������֪ʶ���ʵ�������������


 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
̼��������ҹ���Ҫ�ĵ���Ʒ��֮һ���������������������ӷ���ʧ��Ϊ�˼�����������ȷ�����ʩ����������ⶨ�京������
��ijѧ�������һ���Բⶨ������̼������Ӳⶨ�������ķ���������Ʒ����Բ����ƿ�У�

��1����ѡ���Ҫ��װ�ã���������������˳��Ϊ ��
��2����Һ©���е�Һ�����ʺϵ��� ��
| A��ϡ���� | B��ϡ���� | C��Ũ���� | D���������� |
| �ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 1��20 | 16��21 |
| 2 | 3��00 | 18��90 |
| 3 | 4��50 | 19��49 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʯ����Ҫ�ɷ�ΪCaC2������ΪCaO��CaS���ǹ�ҵ����Ȳ�ij���ԭ�ϣ�ij�о���ѧϰС�������������ַ����ⶨCaC2��������������������и��⡣
����һ����ȡ1.40 g��Ʒ��Բ����ƿ�У��÷�Һ©������������ˮ��ʹ��Ʒ��Ӧ��ȫ������������ɫ
���徭Bϴ����ͨ������װ�ò����Ȳ�������Ϊ��״����448 mL����������Ȳ��ˮ�е��ܽ⣬
��ͬ����
��1����ʯˮ��������Ȳ����Ļ�ѧ����ʽΪ��
��2��������װ���ʵ��Լ��Ĺ��ƿ������˫���������������ɣ��������ʵ���װ�ã�����ͼ�н��䲹����������ע�����ƿ��װ���Լ������ơ�
��3���Ի��ȷ�������������IJ����� ������ţ�
�ټ��װ�õ������ԣ�
�ڻָ������º������µ�����Ͳλ�ã�ʹC��D��Һ����ƽ��
����Aװ�ý�����������һ�����ܣ�ͨ��N2��A��C2H2ȫ�����뵽B��C�У�
�ܶ���ʱ��������Ͳ�ڰ�Һ����͵���ƽ
��4�����������ݿɼ������Ʒ��CaC2����������Ϊ____ ��
����������ȡl.40g��Ʒ����ͼ��ʾʯӢ���У��гּ�����װ��ʡ�ԣ�����a�����ϻ���ͨ���������
������ʯӢ���е���Ʒ����Ӧ��ȫ����ñ���Һ�������ȷ�Ӧǰ������1.80g����Ӧ����ʽΪ��2CaC2+
5O2��2CaO+ 4CO2��
��5����Ӧ��ɺ�ʯӢ������Ʒ����ܱڲ�����ʴ�����û�ѧ����ʽ������ԭ��
��6������ƿ�����Ը��������Һ������Ϊ
��7���ɴ˷�����õ���Ʒ��CaC2�����������ȷ���һ�е�____ �������С������
�ȡ�������ʵ��װ�õĽǶȿ���ԭ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�̷���FeSO4��7H2O��������ɫ����������ˮ�������ư�ȷ��档����������м���������������ʣ������̷���һ�ַ�����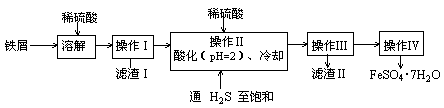
��֪����H2S������Һ�У�SnS������ȫʱ��pHΪ1.6��FeS��ʼ����ʱ��Һ��pHΪ3.0��������ȫʱ��Һ��pHΪ5.5��
��1������������pH��ֽ�ⶨ��ҺpH�IJ����ǣ�
��2�����������������ữ��pH=2��Ŀ����
��3������������Ҫ�IJ����������ձ��� ��
��4��ͨ�����������͵�Ŀ���ǣ��ٳ�ȥ������������Һ�к��е�Sn2+���������ӣ�
��
��5������IV��˳������Ϊ�� ����ȴ�ᾧ�� ��
��6������IV�õ����̷�������������ˮϴ�ӣ���Ŀ���ǣ��ٳ�ȥ������渽�ŵ���������ʣ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ͼ���Ļ������ü������ڽ�Լ��Դ���������ڱ���������ij�о�С��ͬѧ�ԷϾ�п�̸ɵ��Ϊԭ�ϣ����Ͼɵ�غ�п����ת����ZnSO4��7H2O�����̲���ת���ɴ��Ƚϸߵ�MnO2����NH4Cl��ҺӦ���ڻ��������У�ʵ���������£�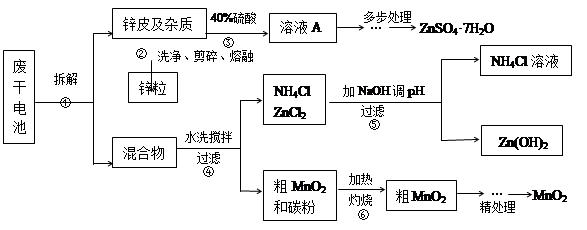
��1�������������õļ�������Ӧѡ ��ѡ�����������������
��2������ҺA�����ĵ�һ���Ǽ��백ˮ����pHΪ9��ʹ���е�Fe3+��Zn2+��������д����ˮ��Fe3+��Ӧ�����ӷ���ʽ ��
��3����������Ϊ�˳�ȥ��Һ�е�Zn2+����֪25��ʱ��
| NH3��H2O��Kb | Zn2+��ȫ������pH | Zn(OH)2���ڼ��pH |
| 1.8��10��5 | 8.9 | ��11 |
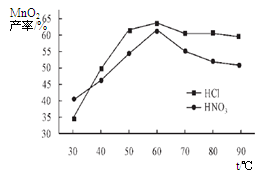
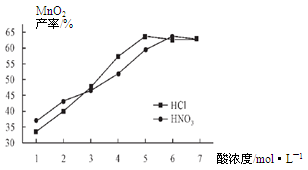
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ��Ӧ��ǰ�������Ļ���ԭ����Դ,�Ӻ�ˮ�п���ȡ���ֻ���ԭ��.��ͼ�ǹ�ҵ�϶Ժ�ˮ�ļ����ۺ����õ�ʾ��ͼ����֪����XΪ��ⱥ��ʳ��ˮ���ã�ĸҺ��±����Ҫ����Ca2+��Mg2+,Cl-,SO42-��Br-������)��ش�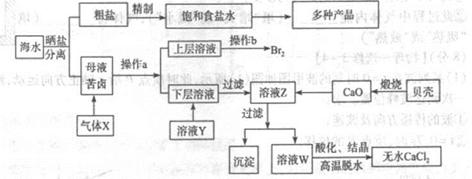
��1���ڴ����к���Ca2+��Mg2+��SO42-�����ʣ�����ʱ���õ��Լ�Ϊ:
| A������ | B���Ȼ�����Һ | C������������Һ | D��̼������Һ��������Լ���˳����(����)______ |

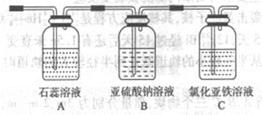
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й�ʵ��ԭ���������ͽ��۶���ȷ����
| A����FeCl3��Һ�еμӹ�����ˮ������ȡFe(OH)3���� |
| B��ȡ������ҺX�������м����������ư�ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2�� |
| C�����������������ӵĻ����Һ�м�������NaOH��Һ�������ú��Һ���ɳ�ȥ������������ |
D����֪I3�� I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
Ϊ�ᴿ�������ʣ�������Ϊ���ʣ�����ѡ�Լ�����������ȷ����
| ѡ�� | ���� | �����Լ� | ���� |
| A | �屽���壩 | CCl4 | ��Һ |
| B | NH3��H2O�� | Ũ���� | ϴ�� |
| C | ���飨��ϩ�� | ��ˮ | ϴ�� |
| D | CO2��SO2�� | Na2CO3������Һ | ϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и���������ֻ��ˮ���ܼ�����ǣ� ��
| A���������ᡢ���Ȼ�̼ | B���Ҵ�����ȩ������ |
| C����ȩ���Ҷ����������� | D�����ӡ��Ҵ������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com