



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ɫ����ˮ���պ���ɫ��dz |
| B����H2��I2������HI��ɵ�ƽ����ϵ��ѹ����ɫ���� |
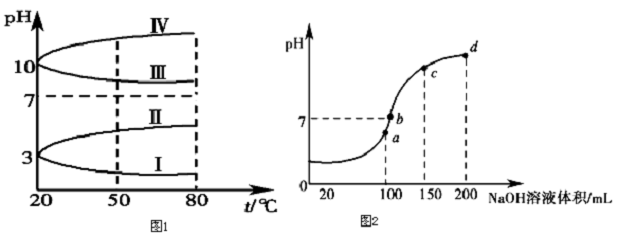
| C���¶ȹ��߶Ժϳɰ����� |
| D����ˮ����ƽ�⣺Br2+H2O?HBr+HBrO��������AgNO3��Һ����Һ��ɫ��dz |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
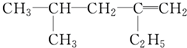 ����������ȷ���ǣ�������
����������ȷ���ǣ�������| A��2��4�һ�4��ϩ |
| B��2�춡��1��ϩ |
| C��2��4����3��ϩ |
| D��4��2�һ�1��ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Y��Z�������һ��Y��Z��������Ϊ7��20�Ļ����� |
| B��X��Y�������ԭ�Ӹ����ȷֱ�Ϊ3��1��2��1�����ֻ����� |
| C��X��Y��Z�������һ���Σ�����X��Y��ZԪ��ԭ�Ӹ�����Ϊ4��2��3 |
| D����X��Y��Z����Ԫ���е�����������ɵľ���10���ӵ���ֻ��2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��100mL 0.4mol/L KNO3��Һ |
| B��100mL 0.2mol/L Cu��NO3��2��Һ |
| C��200mL 0.1mol/L Fe��NO3��2��Һ |
| D��400mL 0.1mol/L Al��NO3��3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ԭ��Ӧ�ı�����Ԫ�ػ��ϼ۷����仯 |
| B����������Ԫ�ػ��ϼ۽��͵ķ�Ӧ��������Ӧ |
| C���û���Ӧһ������������ԭ��Ӧ |
| D�����Ϸ�Ӧ�ͷֽⷴӦ��������������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com