
�ۺ����ú�ˮ�����Ʊ�ʳ�Ρ��������þ�����ʣ�����������ͼ��ʾ��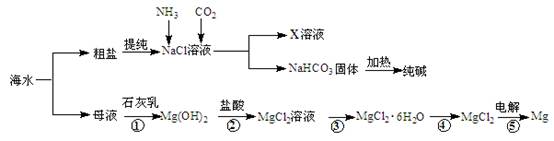
��1����Ӧ�١����У�����������ԭ��Ӧ���� �����ţ���
��2��д����Ӧ�ڵ����ӷ���ʽ ��
��3��X��Һ�е���Ҫ��������Na+�� ��
��4�������к���Na2SO4��MgCl2��CaCl2�ȿ��������ʣ�Ϊ�Ƶô�����NaCl���壬�������£�
���ܽ⣻�����μ��������BaCl2��Һ��NaOH��Һ��Na2CO3��Һ���� ���ܼ���������� �����벹ȫȱ�ٵ�ʵ�鲽�裩
��5�����鴿����Ʒ���Ƿ�NaClӦѡ�õ��Լ��� ��
��1���� ��2�֣�
��2��Mg (OH)2+2H+ = Mg2+ + H2O ��2�֣�
��3��NH4+��2�֣�
��4������ ��2�֣� �������ᾧ�������𰸺���Ҳ���֣���2�֣�
��5��ϡ���ᡢAgNO3��Һ��2�֣�
���������������1����Ӧ�٢����ڸ��ֽⷴӦ�������ڻ��Ϸ�Ӧ�������ڷֽⷴӦ����Ϊ������ԭ��Ӧ����2������кͷ�Ӧ��Mg (OH)2������ˮд�ɻ�ѧʽ��Mg (OH)2+2H+ = Mg2+ + H2O����3���÷�ӦΪ��ˮ��������̼���Ȼ��Ʒ�Ӧ����̼�����Ƴ������Ȼ�泥�X��Һ�е���Ҫ��������NH4+��Na+����4����������ǰ���ɵij������ܽ������ᣬ��Ӧ���˳�ȥ�������ټ����ᣬȻ��ͨ�������ᾧ�õ��Ȼ��ƾ��壻��5��������Ʒ���Ƿ��������ӣ��������ữ����������
���㣺���黯�������л�ѧ֪ʶ�й����⡣


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����÷���м�����������������������ȣ�������ʽ������[Fe(OH)SO4]�Ĺ����������£�
��֪������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Fe(OH)2 | Al(OH)3 |
| ��ʼ���� | 2.3 | 7.5 | 3.4 |
| ��ȫ���� | 3.2 | 9.7 | 4.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
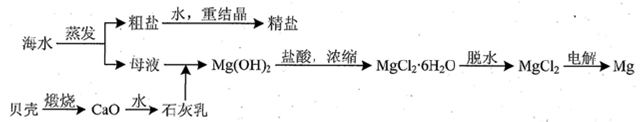
��ˮ�DZ������Ȼ��Դ�����ú�ˮˮ���Եõ�һϵ�в�Ʒ��Ҳ���Խ��з���������
��1�������ȼҵ��Ʒ������SO2���������������£�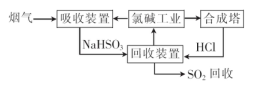
�١�����װ�á��з�����Ӧ�����ӷ���ʽ�� .
������������ѭ�����õ������� ��
��2�����ú�ˮ���������Ч�ؽ��úȼ���ŷŵ�SO2��ɵ�һϵ�л������⡣�乤��������ͼ��ʾ��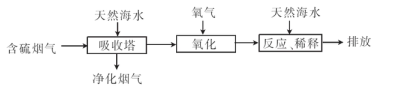
��Ȼ��ˮ���պ������������Ҫ���������������������䷴Ӧԭ���Ļ�ѧ����ʽ�� ��������ĺ�ˮ��Ҫ�����������ƣ���֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��
��3���Ӻ�ˮ�������κ��ĸҺ�к���K+��Na+��Mg2+�������ӣ���ĸҺ����һϵ�еļӹ����Ƶý���þ��
�ٴ����ӷ�ϯ�ĽǶ�˼������ĸҺ�м���ʯ��������������� ��
��Ҫ����MgCl2��6H2O�Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ�� ��
�۵�����ڵ���ˮ�Ȼ�þ���õ�þ�������ض��Ļ�������ȴ��Ϊ����þ�����������п�������þ��������ȴ������ ������ĸ����
A��Ar B��CO2 C ���� D��O2 E��ˮ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������㷺Ӧ������ҩ�ͻ�����ҵ��ij��ѧС���üױ�����Ҫԭ���Ʊ������ᣬ��Ӧ�������£�
�ױ���������ء�������IJ����������ʼ��±���
| ���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | ��ˮ���ܽ��� |
| �ױ� | ��95 | 110.6 | 0.8669 | ���� |
| ������� | 121.5��123.5 |  |  | ���� |
| ������ | 122.4 | 248 | 1.2659 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ����Ҫ���ӵĺ������£�
| �ɷ� | ����/(mg/L) | �ɷ� | ����/(mg/L) |
| Cl- | 18980 | Ca2+ | 400 |
| Na+ | 10560 | HCO3- | 142 |
| SO42- | 2560 | Mg2+ | 1272 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ϸ���������Ҫ�ɷ���V2O5��VOSO4��K2SO4��SiO2��Fe2O3�ȣ������������¹������̻���V2O5��
�ش��������⣺
��1�� VOSO4�У�VԪ�صĻ��ϼ�Ϊ_______�����в����ķ�������Ҫ�ɷ���_________��
��2����ƽ���з�Ӧ�����ӷ���ʽ��
��3��25��ʱ��ȡ������ʵ��������õ��������ʺ���ҺpH֮��Ĺ�ϵ���±���
���ж���ʵ������ʱ�����м��백ˮ������Һ�����pHΪ______________��
��4������ʱ�������е���������Һѭ�����ڢ��е�ˮ�������������������У���ѭ�����õ����ʻ���________________��
��5����ƷV2O5��ͨ�����ȷ�Ӧ����ȡ��������д���÷�Ӧ�Ļ�ѧ����ʽ��______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
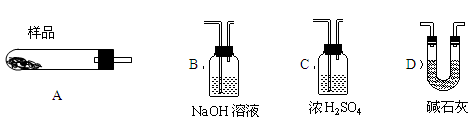
����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�
��1������ʯ��������ܽ����Һ�����Mg2+�⣬�����еĽ��������� ��
��2�����Т����ʱ��������Һ��pH=7~8���й��������������pH���±�����Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ�� �ܽ⣬���� ������
| �������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������棨CeO2����һ����Ҫ��ϡ�������ƽ�������ʾ�����������в��������ķϲ�����ĩ����SiO2��Fe2O3��CeO2�����ʣ���ij�����Դ˷�ĩΪԭ�ϣ���Դ���յĹ����������£�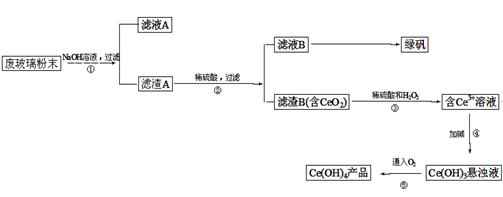
��1��д���ڢٲ���Ӧ�����ӷ���ʽ ��
��2��ϴ������B��Ŀ����Ϊ�˳�ȥ____�������ӷ��ţ�������������Ƿ�ϴ���ķ����� ��
��3��д���ڢ۲���Ӧ�Ļ�ѧ����ʽ_____________________________��
��4���Ʊ��̷�(FeSO4��7H2O)ʱ����Fe2(SO4)3��Һ�м��������м����ַ�Ӧ�����˵õ�FeSO4��Һ���پ� �� �����ˡ�ϴ�ӡ�����Ȳ�������õ��̷���
��5��ȡ���������еõ���Ce(OH)4��Ʒ(��������Ϊ97%)1.0g���������ܽ����0.1000mol/LFeSO4��Һ�ζ����յ㣨�汻��ԭ��Ce3+)������ȷ�μӱ���Һ�����Ϊ mL��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ɫ��ĩ��̼���ơ�����þ������ͭ���Ȼ��ء��Ȼ���е�һ�ֻ�����ɡ�Ϊ�˼����������������ʣ���������ʵ�顣��ȡ���ַ�ĩ����ˮ�ܽ⣬����ɫ��Һ����������Һ�ֳ����ݣ��ֱ����ʵ�飻���ڵ�һ����Һ�еμ�����ϡ���ᣬ�����ݲ�������������Ӧ�����Һ�еμ�AgNO3��Һ�а�ɫ�������ɣ����ڵڶ�����Һ�еμ�����������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ���ֽ�������ɴ˿��жϹ��������п϶��� ��д��ѧʽ����ͬ�����϶�û�� �����ܺ��� ���Կ����е����ʣ��ɲ��� �����飬������и����ʣ��������� ����������йصĻ�ѧ����ʽΪ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com