
��ˮ����Ҫ���ӵĺ������£�
| �ɷ� | ����/(mg/L) | �ɷ� | ����/(mg/L) |
| Cl- | 18980 | Ca2+ | 400 |
| Na+ | 10560 | HCO3- | 142 |
| SO42- | 2560 | Mg2+ | 1272 |
��1��HCO3-+H2O H2CO3+OH-��1�֣�
H2CO3+OH-��1�֣�
��2��Mg(OH)2(1��)��Ca2++ OH-ʮHCO3-��CaCO3��+ H2O (2��)
��3���ٹ��ˣ�1�֣���bd(l��) ����Һ�����γɾ�Ĥ��1�֣�
��4������Һ��죨1�֣� �����ᣨ2�֣���-0.94(2�֣�û�и��Ų��۷�)��
���������������1�������£���ˮ��pH��7.5~8.6֮�䣬��ԭ���Ǵ���̼���������ˮ��ʼ��ԡ�
��2���������������ϲ�����������ʱ�����м����˴������������ӣ�����þ��������Mg(OH)2��ɫ������ͬʱ�ֺ�̼���������̼������ӣ�̼����ֺ����ӽ������CaCO3������CaCO3�����ӷ���ʽ��Ca2++ OH-ʮHCO3-��CaCO3��+ H2O��
��3�����Ȼ��Ƶ��ܽ�����¶ȱ仯����������Ŀ�IJ��ǵõ��ȱ�����Һ�����ǰ�ˮ����ʹ�������������Ӧ�������н϶ྦྷ������ʱΪֹ��ѡ��bd������MgCl2��Һ�õ�MgCl2?6H2O����ʱ��Ҳ��Ҫ������������Ŀ���ǵõ��ȱ�����Һ���ж���Һ�ѱ��͵���������Һ�����γɾ�Ĥ��
��4���ٱ���Mg(OH)2��Һ�еμӷ�̪��������Һ�ʼ��ԣ���������Һ��졣��ȡһ������ĺ�ˮ�����������������̼��������ټ�������NaOH����Mg2+תΪMg(OH)2����25��ʱ������Mg(OH)2��Һ��Ũ��Ϊ5��10-4 mol��L�����1L��Һ��þ���ӵ�����Ϊ5��10-4 *24=1200mg/L��25�棬�÷�����õ�Mg2+���������1272mg/L�ġ���ֵ���ȣ�������ԼΪ��1200-1272��/1272=5.6����
���㣺���⿼��ͼ������������������ԭ��������


 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
±�����Ҫ�ɷ���MgCl2������� Fe3+��Fe2+��Mn2+�����ӡ���±��Ϊԭ�Ͽ��Ƶ���������þ��������������ͼ��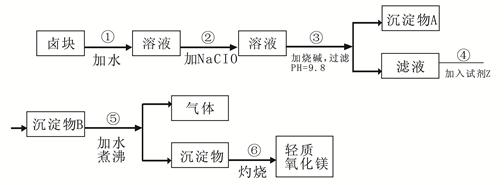
��֪��Fe2+�����������״�����״���Һ�г�ȥ�����Գ���������ΪFe3+������Fe(OH)3������ȥ����Ҫ���Ʒ�����������ʣ�����ݱ�1��2�ṩ�����ϣ���д�հף�
��1 �����������������pH
| ���� | ��ʼ���� | ������ȫ |
| Fe��OH��3 | 2.7 | 3.7 |
| Fe��OH��2 | 7.6 | 9.6 |
| Mn��OH��2 | 8.3 | 9.8 |
| Mg��OH��2 | 9.6 | 11.1 |
| �Լ� | �۸�Ԫ/�֣� |
| ƯҺ����NaClO��25.2%�� | 450 |
| ˫��ˮ����H2O2 ,30%�� | 2400 |
| �ռ��98% NaOH�� | 2100 |
| �����99.5% Na2CO3�� | 600 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һ���ḻ����Դ���⣬ͨ����ˮ���ۺ����ÿɻ���������ʹ�����ʹ�á�
��1�� ��ˮ���εĿ������ã�
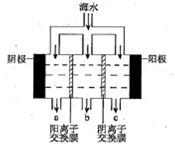
��.��ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���뺣�ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ�_______�ء�
II.Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ������____________________________________________����дһ�㼴�ɣ�
��2�����������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ������ͼ��ʾ����ش��������⣺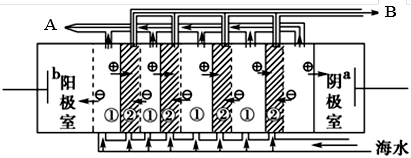
��.��ˮ����ֱ��ͨ�뵽��װ���У�������_____________________________________________��
��. B���ų�����________(���ˮ����Ũˮ��)��
��3���ÿ�±����Na+��K+��Mg2+��Cl-��Br-�����ӣ�����ȡ�壬�������������£�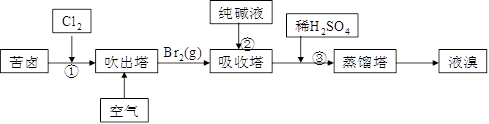
��.���������е���Һ��BrO3�������������з�Ӧ�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣ�
_________________________________________��
��.ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ�����»�ú�Br2����Һ��_____________________________________________________________________��
��.����������ͨ��ˮ�������ȣ������¶���900C���ҽ��������ԭ����___________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ͭ���к�����Ҫ����ΪFe2+��Al3+�ȣ���������CuSO4��5H2O��Ҫ������������ͼ��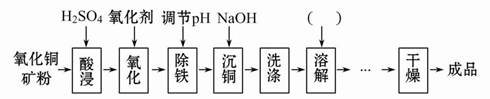
(1)ͼ�м�����������Ŀ���� ������±��ش������������pH��ΧΪ ��
(2)��������ѡ��KMnO4���ή�Ͳ�Ʒ�Ĵ��ȣ���ԭ���� ��ʵ��������NaClO��Ϊ�����������������Ӧ�����ӷ���ʽΪ ��
(3)������ͼ��������ȱ���輰�������Լ��Ļ�ѧʽ���ɹ�ѡ��IJ����У���ȡ������Ũ�������ˡ�ϴ�ӡ����½ᾧ������Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮAlCl3�������л��ϳɵĴ�����ʳƷ���ɼ��ȡ���ҵ������������Ҫ�ɷ���A12O3��Fe2O3����ʯ�ͽ�����Ҫ�ɷ���C������ͼ��ʾ���̽���һϵ�з�Ӧ���Ʊ���ˮAlCl3��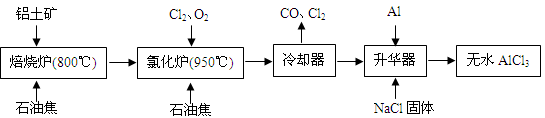
��1���Ȼ����ڼ�������������������̬�Ȼ����Ļ�ѧʽΪAl2Cl6��ÿ��Ԫ�ص�ԭ���������ﵽ8�����ȶ��ṹ����AlCl3�ǣ� ���壬��ṹʽΪ�� ��
��2���Ȼ�¯��Al2O3��Cl2��C��Ӧ�Ļ�ѧ����ʽ�� ��
��3����ȴ���ų���β���к��д���CO������Cl2������Na2SO3��Һ��ȥCl2���˷�Ӧ�����ӷ���ʽΪ�� ��
��4������������Ҫ����AlCl3��FeCl3�����������Al���������ǣ� ��
��5��AlCl3��Ʒ��FeԪ�غ���ֱ��Ӱ����Ʒ�ʣ�Ϊ�ⶨ��Ʒ��FeԪ�صĺ������ֳ�ȡ16.25g��ˮAlCl3��Ʒ�����ڹ�����NaOH��Һ�����˳�����������ᆳϴ�ӡ����ա���ȴ�����ز�����������Ϊ0.32g�����Ʒ��FeԪ�صĺ���Ϊ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
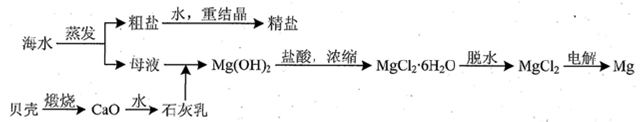
�ۺ����ú�ˮ�����Ʊ�ʳ�Ρ��������þ�����ʣ�����������ͼ��ʾ��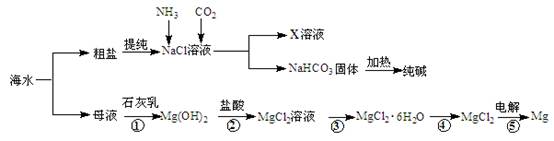
��1����Ӧ�١����У�����������ԭ��Ӧ���� �����ţ���
��2��д����Ӧ�ڵ����ӷ���ʽ ��
��3��X��Һ�е���Ҫ��������Na+�� ��
��4�������к���Na2SO4��MgCl2��CaCl2�ȿ��������ʣ�Ϊ�Ƶô�����NaCl���壬�������£�
���ܽ⣻�����μ��������BaCl2��Һ��NaOH��Һ��Na2CO3��Һ���� ���ܼ���������� �����벹ȫȱ�ٵ�ʵ�鲽�裩
��5�����鴿����Ʒ���Ƿ�NaClӦѡ�õ��Լ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������;�㷺����ҵ�ϳ��ú�����Al2O3�ķ�����FeO��V2O5�����۷���ȡV2O5����Ҫ�������£�
��֪���ٱ���ʱ�ɷ�����Ӧ��V2O5 + Al2O3+2Na2CO3 2NaVO3 +2NaAlO2 +2CO2
2NaVO3 +2NaAlO2 +2CO2
�ڳ��������ʵ��ܽ�ȣ�NaVO3��21��2 g /100gˮ��HVO3��0��008 g /100gˮ
��1����������B������Ҫ�ɷ��� ����д��ѧʽ��
��2�������У���ֱ����H2SO4���ݡ�����A����ȡHVO3��ԭ���� ��
��3���������١����� ��ϴ�ӡ������ϴ�ӣ����Ʒ�п��ܺ��еĽ����������� �� ������װ�ã����ּг�����ʡȥ��������ʵ���ҽ��С������ڡ����� ��������ţ�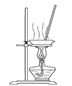

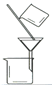
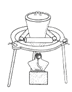
A B C D
��4��NaVO3����ԭ�͵�����������V2O5����NaOH��Һ����ȡ����Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾʵ�������գ�
��1��д��������Ӧ�����ӷ���ʽ��
�� ��
�� ��
�� ��
��2����գ�a b c d ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ȼ���е����ʶ������Ի�������ʽ���ڣ������ǻ����ķ�����ᴿ������գ�
��1����ȥNaCl�����л��е�����CaCO3�������е�ʵ�����Ϊ�� �� ���������ᾧ��
��2����ȥNaCl�е�Na2SO4�����μ������ҺΪ(�����ʻ�ѧʽ)�� �� �� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com