
������һ���ḻ����Դ���⣬ͨ����ˮ���ۺ����ÿɻ���������ʹ�����ʹ�á�
��1�� ��ˮ���εĿ������ã�
��.��ˮ����Ŀǰ�����Ϊ�������������ѡ��Զ�뽭���뺣�ڣ�������꣬��ϫ��������ƽ̹�տ��ĺ�̲�����������Ϊ��ˮ�ء������غ�_______�ء�
II.Ŀǰ��ҵ�ϲ��ñȽ��Ƚ������ӽ���Ĥ���۷������ȼҵ�������ڵ����������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ������˵���ȼ������������ӽ���Ĥ������____________________________________________����дһ�㼴�ɣ�
��2�����������ǽ�������չ������һ�ֽϺõĺ�ˮ������������ԭ������ͼ��ʾ����ش��������⣺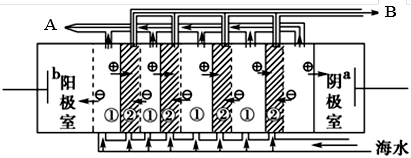
��.��ˮ����ֱ��ͨ�뵽��װ���У�������_____________________________________________��
��. B���ų�����________(���ˮ����Ũˮ��)��
��3���ÿ�±����Na+��K+��Mg2+��Cl-��Br-�����ӣ�����ȡ�壬�������������£�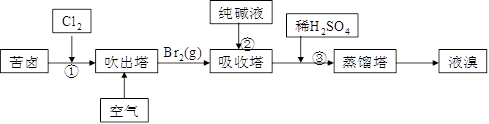
��.���������е���Һ��BrO3�������������з�Ӧ�����ӷ���ʽΪ�ߣߣߣߣߣߣߣߣߣߣߣߣ�
_________________________________________��
��.ͨ�����Ȼ��ѻ�ú�Br2����Һ��Ϊ�λ��辭�����������ա��ữ�����»�ú�Br2����Һ��_____________________________________________________________________��
��.����������ͨ��ˮ�������ȣ������¶���900C���ҽ��������ԭ����___________________________________________________________________________��
��1�� �� �ᾧ �� ��ֹH2��Cl2������Ӧ����������ը����ֹCl2�����ɵ�NaOH��Һ��Ӧ��ʹ�ռ��Ʒ������ ������������Ҳ�Ʒ֣�
��2���� ��ˮ�к��϶�Mg2+ ��Ca2+�������ӣ����ʱ�����Mg(OH)2��Ca(OH)2�ȳ����Ӷ����������ӽ���Ĥ �������𰸾��Ʒ֣� ��3�֣� �� ��ˮ
��3����3CO32��+ 3Br2 �� 5Br��+BrO3��+3CO2��
���壬���Br2��Ũ�� �������𰸾��Ʒ֣�
���¶ȹ������Խ�Br2�������������¶ȹ����ֻὫ������ˮ��������������𰸾��Ʒ֣�
���������������1���� �δ���ˮ���о��������أ��õ�Ũ�Ƚϴ��±ˮ���ٽ�±ˮת�Ƶ��ᾧ���нᾧ�����յô��Σ��������ӽ���Ĥֻ����������ͨ������ֹ�����Ӻ�����ͨ�����Ӷ��������������������������������������Ͳ�����Ӵ���Ӧ��������ը��ͬʱ�����ϲ�������������Ҳ������������Ӧ�����Ƚϸߣ�
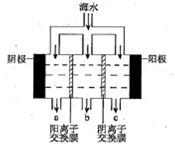
��2���� ��ˮ�и���Ca2+��Mg2+���ӣ�������ˮֱ��ͨ�뵽��װ���У���ˮ�е�Ca2+��Mg2+�����������������ӽ�ϲ���Mg(OH)2��Ca(OH)2�ȳ����Ӷ����������ӽ���Ĥ �� ͼ�Тڴ���ֱ���糡�������£���Һ�е����Ӿ�������Ǩ�ơ���Ĥֻ����������ͨ�����������ӽ�����������Ĥֻ����������ͨ�����������ӽ������������ʹ��ЩС�ҵ�һ���ֱ�ɺ����Ӻ��ٵĵ�ˮ�ң���ˮ��Ϊ��ˮ�����뵭ˮ�����ڵ�С�����ɾۼ��������ӵ�Ũˮ�ң���ˮ��ΪŨˮ������B����ˮΪ��ˮ��
��3������̼���ƣ����巴Ӧ��BrO3�����ɣ���Ӧ�����ӷ���ʽΪ3CO32��+ 3Br2 �� 5Br��+BrO3��+3CO2��
��Ӣٳ�������Һ����ĺ������ߣ����ֱ������Ʒ�ɱ��ߣ�������Ҫ��һ��Ũ���壬������Ũ�ȣ�
�� �¶ȹ���ˮ�������������к���ˮ�֣��¶ȹ����岻����ȫ���������ʵ͡�
���㣺���麣ˮ���ۺ����á���ˮɹ�Ρ���������������ˮ���Ӻ�ˮ�������ԭ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣����������Ҫ�ɷֿɱ�ʾΪFeO��Cr2O3��������MgO��Al2O3��Fe2O3�����ʣ��������Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7��������ͼ��
��֪����4FeO?Cr2O3+8Na2CO3+7O2 8Na2CrO4+2Fe2O3+8CO2����
8Na2CrO4+2Fe2O3+8CO2����
��Na2CO3+Al2O3 2NaAlO2+CO2����
2NaAlO2+CO2����
��Cr2CO72-+H2O 2CrO42-+2H+
2CrO42-+2H+
��������ش��������⣺
��1������X����Ҫ���� ����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4��5��Ӧ��ʹ�� ______________ ����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH��5����Ŀ���� _______________________ ��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� �����ˡ� �����
��4���±���������ʵ��ܽ�����ݣ�����III������Ӧ�Ļ�ѧ����ʽ�ǣ�Na2Cr2O7 + 2KCl��K2Cr2O7 ��+ 2NaCl���÷�Ӧ����Һ���ܷ�����������_____________________________��
| ���� | KCl | NaCl | K2Cr2O7 | Na2Cr2O7 | |
| �ܽ�ȣ�g/100gˮ�� | 0�� | 28 | 35��7 | 4��7 | 163 |
| 40�� | 40��1 | 36��4 | 26��3 | 215 | |
| 80�� | 51��3 | 38 | 73 | 376 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������(CeO2)��һ����Ҫ��ϡ�������ƽ�������ʾ�����������в��������ķϲ�����ĩ(��SiO2��Fe2O3��CeO2�Լ���������������ϡ�������)��ij�������Դ˷�ĩΪԭ�ϻ����棬���ʵ����������: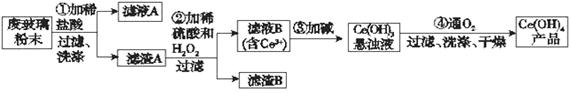
��1��ϴ������A��Ŀ����Ϊ�˳�ȥ____________�������ӷ��ţ�������������Ƿ�ϴ���ķ�����_________________________________________________________��
��2���ڢڲ���Ӧ�����ӷ���ʽ��______________________________________������B����Ҫ�ɷ���___________________��
��3����ȡ�Ƿ���ϡ��Ԫ�صij��÷�������֪������TBP��Ϊ��ȡ���ܽ������Ӵ�ˮ��Һ����ȡ������TBP______________����ܡ����ܡ�����ˮ���ܡ�ʵ���ҽ�����ȡ����ʱ�õ�����Ҫ����������_______________���ձ�������������Ͳ�ȡ�
��4��ȡ���������еõ���Ce(OH)4��Ʒ0.536 g���������ܽ����0.100 0 mol��L��1 FeSO4����Һ�ζ����յ�ʱ���汻��ԭΪCe3����������25.00 mL����Һ���ò�Ʒ��Ce(OH)4����������Ϊ___________�����������λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ϡ��Ԫ�������ڱ��Т� B���֡��ƺ���ϵԪ�ص��ܳƣ����Ƕ��Ǻܻ��õĽ��������ʼ�Ϊ���ƣ��������ϼ�Ϊ+3�������ƣ�Y��Ԫ���Ǽ���ͳ�������Ҫ���ϡ��ҹ��̲��ŷḻ���ƿ�ʯ�� Y2FeBe2Si2O10�����Դ˿�ʯΪԭ�����������ƣ�Y2O3������Ҫ�������£�
��֪��I���йؽ��������γ������������ʱ��pH���±���
| | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe3+ | 2.7 | 3.7 |
| Y3+ | 6.0 | 8.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Һ�žú���������������AgN3��������ը��ֱ���ŷŻ���Ⱦ���������������Դ���˷ѡ�ij�о�С������˴�������Ӧ��ķ�Һ�У���������������Һ�����費��������������������������ʵ�����̣�
����֪��[Ag��NH3��2]������Һ�д���ƽ�⣺[Ag��NH3��2]��??Ag����2NH3��
��1��д�������ڢٲ���Һ��ϡHNO3��Ӧ�����ӷ���ʽ ��
��2�������ڢڲ����������Ҫ������Ŀ���� ��
�������̿��ܲ����Ĵ�����Ⱦ���� ��
��3���ҷ��������յõ����۵�����ƫ���ų�δϴ�Ӹɾ������أ����ܵ�ԭ���� ��
��4��ʵ��������������Һ�IJ��������� ��
��5����֪�ҷ����ڢ۲���Ӧ��H2S��������������յõ�����21.6 g��������������ʧ�������ϸò���Ҫ�������� g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ�DZ������Ȼ��Դ�����ú�ˮˮ���Եõ�һϵ�в�Ʒ��Ҳ���Խ��з���������
��1�������ȼҵ��Ʒ������SO2���������������£�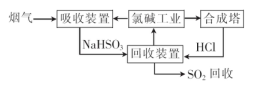
�١�����װ�á��з�����Ӧ�����ӷ���ʽ�� .
������������ѭ�����õ������� ��
��2�����ú�ˮ���������Ч�ؽ��úȼ���ŷŵ�SO2��ɵ�һϵ�л������⡣�乤��������ͼ��ʾ��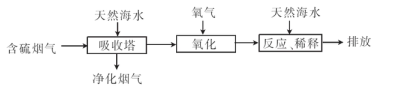
��Ȼ��ˮ���պ������������Ҫ���������������������䷴Ӧԭ���Ļ�ѧ����ʽ�� ��������ĺ�ˮ��Ҫ�����������ƣ���֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��
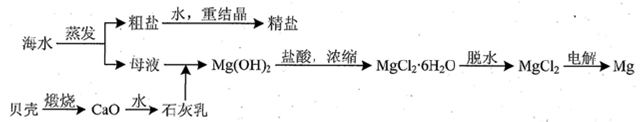
��3���Ӻ�ˮ�������κ��ĸҺ�к���K+��Na+��Mg2+�������ӣ���ĸҺ����һϵ�еļӹ����Ƶý���þ��
�ٴ����ӷ�ϯ�ĽǶ�˼������ĸҺ�м���ʯ��������������� ��
��Ҫ����MgCl2��6H2O�Ƶ���ˮ�Ȼ�þ��Ӧ��ȡ�Ĵ�ʩ�� ��
�۵�����ڵ���ˮ�Ȼ�þ���õ�þ�������ض��Ļ�������ȴ��Ϊ����þ�����������п�������þ��������ȴ������ ������ĸ����
A��Ar B��CO2 C ���� D��O2 E��ˮ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������ҡ������Դ�ı��⡣�й�Ҫʵʩ����ǿ��ս�ԣ�ʵ���ɺ���������ǿ�����������롣�����Ѿ���Ϊ�����ҹ����÷�չ����Ҫ���棬��ˮ���ۺϿ����������Ǻ��õ�һ���֣���ˮ�п���ȡ���ֻ���ԭ�ϣ������ǹ�ҵ�϶Ժ�ˮ�ļ����ۺ����õ�ʾ��ͼ������������ͼ��ʾ��
��1��д���١��ڷ�Ӧ�����ӷ���ʽ��
��______________________����______________________��
��2����ҵ�����õ�ⱥ��ʳ��ˮ������������������ȡ���ᣬΪ��������ɫ��ѧ���ʹ������ַ�Ӧ����ȡ��������������ȼ�յİ취���ɱ�������ȼ�ղ���ȫ��Ⱦ��������д��������������ȼ�յ�ʵ������______________________��
��3�������к���Ca������Mg������SO4���������ʣ����ƺ�ɵõ�����NaC1��Һ���������г����Լ���A������ B������������Һ C��̼������Һ������ʱ������������Լ�����ȷ˳����______________��������ţ�
��4������þ�ڿ�����ȼ��ʱ��������MgO�⣬��������Mg3N2���ɡ��ѵ����ʵ����Ľ���þ�ֱ���ڣ�A����������O2���У�B��������̼�����У�C�������С���ȫȼ�պõ��Ĺ������ʵ������ɴ�С��˳����______________��������ţ�
��5������ⱥ��NaCl��Һ���ɵ�����ͨ������������Һ�п��Եõ�NaClO��ij��ѧ��ȤС��̽��NaClO������CO(NH2)2�ķ�Ӧ���ͨ��ʵ�鷢�ֲ����ij�����⣬������ﶼ���ܲ������ѭ�������ʣ���÷�Ӧ�Ļ�ѧ����ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ˮ����Ҫ���ӵĺ������£�
| �ɷ� | ����/(mg/L) | �ɷ� | ����/(mg/L) |
| Cl- | 18980 | Ca2+ | 400 |
| Na+ | 10560 | HCO3- | 142 |
| SO42- | 2560 | Mg2+ | 1272 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�Ի�����Ϊԭ��������������������к�Fe2O3��SiO2��Al2O3��MgO�ȡ�ʵ����ģ�ҵ���������Ʊ�����(Fe2O3)���������£�
��1���������ijɷ�������������������� �� д�����ܹ���Fe2O3��ϡ���ᷴӦ�����ӷ�Ӧ����ʽ�� ��
��2�����������У�Ϊ��ȷ������Ĵ��ȣ�����������Ҫ������Һ��pH�ķ�Χ��_________��������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com