
����Ŀ�������������ǿ�ȸߡ���ʴ�Ժá������Ըߵ��ص�����㷺���ڸ�������
(1)��̬ Ti ԭ�Ӻ�������Ų�������ܼ�������____������ͬ���ڵ�Ԫ���л�̬ԭ��δ�ɶԵ�����������ͬ����__________�֡�
(2)�ѱȸ��ᣬ����Ӳ����һ�����˵Ľṹ���ϡ���Ӳ�ȱ������ԭ����_____��
(3)����Ľṹ���� M ��ṹ��ͼ��ʾ��
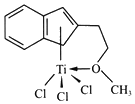
����ɸ������Ԫ���У��縺��������___________(��Ԫ�ط���)
��M���_________(����)
a.���� b.���� c.��λ�� d.���Ӽ� e.���
(4)TiO2������Ũ���Ტ����һ�����Ӿ��壬��֪����������������״�ۺ�����ʽ���ڵ����������ӣ���ṹ��ͼ��ʾ���仯ѧʽ��______ д��һ����������![]() ��Ϊ�ȵ�����ķ���______ ��
��Ϊ�ȵ�����ķ���______ ��

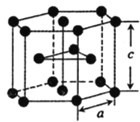
(5)�ѵ��ʵľ�����ͼ��ʾ���þ���Ϊ______�ѻ�(��ѻ���ʽ)����ԭ�ӵ���λ����_______����֪����������a=0.295nm��c=0.469nm������Ѿ�����ܶ�Ϊ___________g��cm-3(NA��ʾ�����ӵ���������ֵ���г�����ʽ����)
���𰸡�3d 3 Ti ԭ�ӵļ۵�������Al �࣬��������ǿ O de ![]() ��TiO2+ CCl4(SiCl4) �������� 12
��TiO2+ CCl4(SiCl4) �������� 12 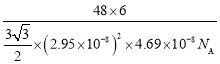 ��
��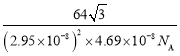
��������
(1)TiԪ��Ϊ22��Ԫ�أ���̬��ԭ�ӵĺ�������Ų�Ϊ1s22s22p63s23p63d24s2��������ܼ�������3d����ԭ�Ӻ���δ�ɶԵ�����Ϊ2������ͬ����Ԫ���У���̬ԭ�ӵ�δ�ɶԵ�����������ͬ��ԭ�ӵļ۲�����Ų�������Ϊ3d74s2��4s24p2��4s24p4����Ni��Ge��Se����3�֣�
(2)Ti ԭ�ӵļ۵�������Al �࣬��������ǿ��������Ӳ�ȱ�����
(3)����ɸ����ʵ�Ԫ����H��C��O��Cl��Ti���ǽ�����Խǿ���縺��Խ������Ԫ���зǽ�������ǿ����OԪ�أ�����OԪ�صĵ縺�����
�ڷ����к���̼̼������̼��������̼�ⵥ���Ⱦ�Ϊ������̼̼˫������������Oԭ�Ӻ�Tiԭ��֮���γ���λ����Clԭ�Ӻ�Tiԭ��֮��Ϊ���ۼ�������M�в������Ӽ������������ѡde��
(4)��ͼ��ÿ��Tiԭ������Oԭ�Ӹ���=2��![]() =1����Ti��Oԭ�Ӹ���֮��Ϊ1��1���仯ѧʽΪ
=1����Ti��Oԭ�Ӹ���֮��Ϊ1��1���仯ѧʽΪ![]() (��TiO2+)��
(��TiO2+)��![]() ��ԭ�Ӹ���Ϊ5���۵�������Ϊ32�����以Ϊ�ȵ��ӵķ�����CCl4��SiCl4�ȣ�
��ԭ�Ӹ���Ϊ5���۵�������Ϊ32�����以Ϊ�ȵ��ӵķ�����CCl4��SiCl4�ȣ�
(5)��ͼ��֪�þ���Ϊ�������ܶѻ�����������ԭ��Ϊ����������ڲ���9����ԭ�Ӿ��������������ȣ��ڸþ������Ϸ��������ڲ�����3��һ������ԭ�ӣ�������ԭ�ӵ���λ��Ϊ12���������е���ԭ�Ӹ���Ϊ![]() =6�����Ծ���������m=
=6�����Ծ���������m=![]() g���þ�������Ϊ�������Σ����Ϊa�����Ե����Ϊ3a2��sin60�����������V=3a2��sin60���c=
g���þ�������Ϊ�������Σ����Ϊa�����Ե����Ϊ3a2��sin60�����������V=3a2��sin60���c=![]() �����Ծ�����ܶ�Ϊ
�����Ծ�����ܶ�Ϊ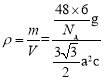 ����a=0.295nm��c=0.469nm����ɵþ�����ܶ�Ϊ
����a=0.295nm��c=0.469nm����ɵþ�����ܶ�Ϊ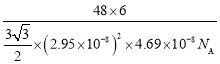 ��
��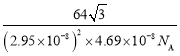 ��
��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��SO2��ʹ����KMnO4��Һ��ɫ����ӦΪ2KMnO4+5SO2+2H2O=2MnSO4+K2SO4+2H2SO4
��1����˫���ţ������ţ�������з�Ӧ�е���ת�Ƶķ������Ŀ��___
2KMnO4+5SO2+2H2O=2MnSO4+K2SO4+2H2SO4
��2���÷�Ӧ�л�ԭ����___����ԭ������___��
��3������1molKMnO4��ȫ��Ӧ����ת�Ƶĵ��ӵ����ʵ�����___mol���μӷ�Ӧ��SO2�����״�������Ϊ___L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪NaBiO3Ϊdz��ɫ���ɫ������NaBiO3�������ữ��MnSO4��Һ������Ӧ��5NaBiO3��2Mn2����14H����2MnO![]() ��5Bi3����5Na����7H2O�������ƶ���ȷ����
��5Bi3����5Na����7H2O�������ƶ���ȷ����
A.������Ӧ�е�MnSO4��ҺҲ����������������ữ
B.����BiO![]() ����Na+�Ĵ���
����Na+�Ĵ���
C.��������Ӧ��֪�����ԣ�NaBiO3��HNO3��KMnO4
D.NaBiO3��Ũ���ᷴӦ�����ӷ���ʽΪ��NaBiO3+2Cl��+6H��=Bi3++Na++3H2O+Cl2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2020��7��1������ȫ����Χ��ʵ���������������ŷű����ñ��Ե������̼�⻯��(HC)��һ����̼���������ӵ��ŷ�������ƽ���Ϊ�Ͽ���
(1)��ȼ��(A/F:����������ȼ������֮��)��β���ŷš������������ȶ��кܴ�Ӱ�졣�������������ⶨ���ҵ���ѿ�ȼ������(������ͼ1)����ͼ��֪��ѿ�ȼ������Ϊ___������������________���ƹ�ʹ����Ǧ���͵��ŵ���____________��

(2)����β��ͨ����Ԫ��װ�þ�����ԭ���ǣ�2NO(g)+2CO(g)![]() 2CO2(g)+N2(g) ��H1
2CO2(g)+N2(g) ��H1
��֪���� N2(g)+O2(g)��2NO(g) ��H2
�� C(s)+O2(g)��CO2(g) ��H3
�� C(s)+![]() O2(g)��CO(g) ��H4
O2(g)��CO(g) ��H4
���H1��_______(�ú���H2����H3����H4�Ĺ�ϵʽ��ʾ)��
(3)ѡ���Դ���ԭ����(SCR)���ð������ؽ�NOx��ԭΪN2��H2O��ԭ���ǣ�NO(g)+NO2(g)+2NH3(g) ![]() 3H2O(g)+2N2(g) ��H
3H2O(g)+2N2(g) ��H
��������������ʱ��NO��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ2��ʾ�����H_____0��p1 ____ p2(����������������)��
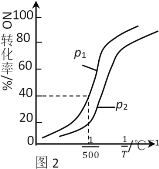
����500��ʱ��2L�ܱ������г���1molNO��1molNO2��2molNH3����ƽ��ʱѹǿΪp1MPa����500��ʱ�÷�Ӧ��ƽ�ⳣ��Kp��_______��
(4)����β���л�����NH3�ȣ�����������㷺Ӧ���ڻ��������ʡ���ҩ������
����֪N2H4��ˮ��Һ�е�һ�����뷽��ʽΪN2H4H2O![]() +OH������
+OH������![]() �ĵ���ʽΪ________________��
�ĵ���ʽΪ________________��
����֪��Ag+(aq) +2NH3(aq)[Ag(NH3)2]+(aq)����ƽ�ⳣ������ʽΪKf ��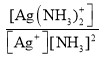 ��1.7��107��Ksp(AgCl)��1.7��10��10����1Lc molL��1�İ�ˮ���ܽ�0.1mol AgCl(s) (������Һ�����Ϊ1L)����ð�ˮ����СŨ��c��_____molL��1(������λ��Ч���֣���ʾ��
��1.7��107��Ksp(AgCl)��1.7��10��10����1Lc molL��1�İ�ˮ���ܽ�0.1mol AgCl(s) (������Һ�����Ϊ1L)����ð�ˮ����СŨ��c��_____molL��1(������λ��Ч���֣���ʾ��![]() ��3.16)��
��3.16)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪NA�ǰ����ӵ�������ֵ������˵����ȷ����
A.100 g46%�ļ���ˮ��Һ����������ԭ����ĿΪ 5NA
B.�����£�1L pH=7 �� lmolL-1 CH3COONH4 ��Һ�� CH3COO-��![]() ��Ŀ��ΪNA
��Ŀ��ΪNA
C.11 g��![]() H��
H��![]() O��ɵij���ˮ�У����е�������ĿΪ 5NA
O��ɵij���ˮ�У����е�������ĿΪ 5NA
D.������ ��5.6gFe ��������ˮ������Ӧ��ת�Ƶ�����Ϊ0.3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ˮ��������ϵ����ϵ��ÿ���˵Ľ���������һ��Ũ�ȵ�NO3-�������ཡ������Σ����NO3-����������Ѫ�쵰���е�Fe��II����ʹ��ʧȥЯ������,Ϊ�˽�������ˮ��NO3-��Ũ�ȣ�ij��ȤС�������ͼ������
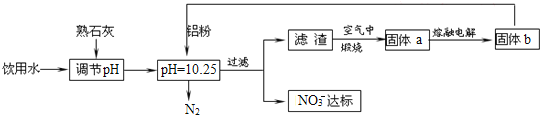
��ش��������⣺
��1������ѪҺ�����ȱ���������ӣ��ͻ����ȱ����ƶѪ���г����۵�ij����Ƭ�к�����������ϸС�Ļ�ԭ���ۣ���Щ����������θ�ᣨθ�����Ҫ�ɷ���HCl��������ת���������Ρ�д���÷�Ӧ�����ӷ���ʽ��____��
��2����֪���˺�õ���������һ�ֻ���������Һ�����ۺ�NO3-��Ӧ�����ӷ���ʽΪ__��
��3���÷�����ѡ����ʯ�ҵ���pH��������___��___��
��4����H2����ԭ��Ҳ�ɽ�������ˮ��NO3-��Ũ�ȣ���֪��Ӧ�еĻ�ԭ���������������ɲ������ѭ���������ԭ�������ӷ���ʽΪ___��
��5������ˮ�е�NO3-��Ҫ������NH4������֪���������õ������£�NH4������������Ӧ��������NO3-��������Ӧ�������仯ʾ��ͼ��ͼ����д��1molNH4��(aq)ȫ��������NO3-(aq)���Ȼ�ѧ����ʽ��____��
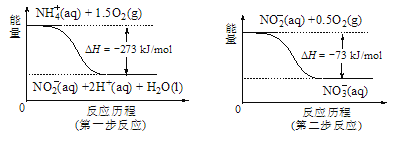
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������.ijͬѧ����CO2��Na2O2��Ӧ��̽��ʵ��(��ʵ�����漰�����ݻ�������ͬ״���²ⶨ)����ش���������:
(1)����ͼװ���Ʊ�������CO2.
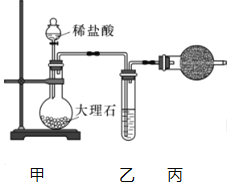
�ٱ�װ�õ�������_______����װ����ʢװ���Լ���_______.
����CO2�л���HCl����HCl��Na2O2������Ӧ�Ļ�ѧ����ʽΪ_________��
(2)����ͼ��ʾװ�ý���ʵ��(�г�װ����)��
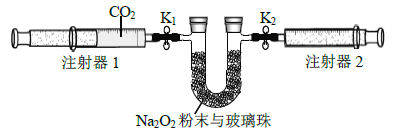
������װ����.Ȼ��________.����ע����1��ȡ100 mL������CO2,����������K1��,ע����2�Ļ����Ƶ���������K2������֧U�ι���װ��������Na2O2��ĩ�벣���顣
�ڴ�ֹˮ��K1��K2,�����ƶ�ע����1�Ļ������ɹ۲쵽��������________��
��ʵ�������.�軺������CO2,��Ŀ����__________.Ϊ�ﵽ��ͬĿ�ģ����ɽ��еIJ�����__________________________��
(3)ʵ�������ע����1�Ļ����Ƶ���ʱ�����ע����2����������Ϊ65 mL����CO2��ת������_______________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ͬ��Ba(OH)2��Һ�У��ֱ�������ʵ���Ũ����ȵ�H2SO4��NaHSO4��Һ���䵼�������������Һ����仯����������ͼ��ʾ�����з�������ȷ����
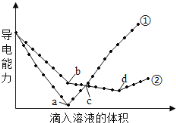
A.�ٴ����μ�H2SO4��Һ�ı仯����
B.b�㣬��Һ�д������ڵ�������Na+��OH-
C.a��d�����Ӧ����Һ��������
D.c�㣬����Һ�к�����ͬ���ʵ�����OH-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(H2SeO3)Ҳ��һ�ֶ�Ԫ���ᣬ��������һ����ɫ���壬������ˮ���н�ǿ�������ԡ�
(1).д����������뷽��ʽ_______________________��
(2).���������������ع��ȿ��Ƶ�����(H2SeO4)����ƽ�÷�Ӧ����ʽ___�����õ����ŷ��������ת�Ƶķ������Ŀ___��
H2SeO3 + KMnO4 �� K2SeO4+ MnSeO4+ H2SeO4+
����ͬ���Ԫ��Te������������ˮ��������(H6TeO6)�����Ա�H2SO4______(ѡ�ǿ��������)���������Ա�����ǿ��
��������ͨ��SO2���壬����Ӧ�����ɵ�TeO2��Te�����ʵ���֮��Ϊ2��1��д���÷�Ӧ�Ļ�ѧ����ʽ_________________����6mol������һ����SO2ǡ����ȫ��Ӧ��������Һ���Ϊ20L����������Һ��pHΪ______��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com