
����Ŀ����һNa2SO3�����ֱ����������ʡ�Ϊ�ⶨ��Ʒ���ȣ��ס�����λͬѧ�ֱ����������ʵ�鷽����
����ͬѧ����ȡm g��Ʒ����ˮ�����������BaCl2��Һ�����ˣ�������м���������ᣬ�ٹ��ˣ�����������ϴ�Ӻ���к�ɣ���ȴ��Ƶ�����Ϊm1 g��
(1)BaCl2��Һ���������ԭ����___________________________________________������Һ�еμ�____________��Һ����û�а�ɫ�������ɣ���֤��BaCl2��Һ���㣬�����������Һ�м���BaCl2��Һ����й��ˡ�ϴ�Ӳ�������ķ�����__________________________________________________����ϴ��Һ�еμ�_______________��Һ�������жϲ��������Ƿ�ϴ�Ӹɾ���
(2)�жϲ�����������m1 g�ܹ���Ϊʵ��ⶨֵ�������ʽ��������Ʒ���ȣ����벹���ʵ�������_________________________________________________________��
����ͬѧ����ȡm g��Ʒ����ˮ�������Һ���õζ���ȡV mL����ƿ�У���Ũ��Ϊc mol/L�ı����Ը��������Һ�ζ����յ㡣��Ӧ��ϵΪ��SO32�� + MnO4�� SO42�� + Mn2+ (δ��ƽ)
(3)������Ʒ��Һʱ����һ����Ҫ��ʵ��������__________(ѡ��𰸱��)��
a������ƿ b�������� c���ζ��� d���ձ�
(4)�ζ��յ���ж�������___________________________________________________��
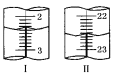
(5)�����ζ��У��ζ�����ע�����Ը��������Һ֮ǰ����������ˮϴ��������__________________________________________���ζ���Һ��仯��ͼ��ʾ����ͼ��ͼ����ʾ���ĵĸ��������Һ���Ϊ__________________��
���𰸡�ȷ��SO42-������ȫ Na2CO3 �ڹ������м�ˮ����û���壬ʹ����Ȼ���£��ظ�2~3�� AgNO3 �ظ���ɡ���ȴ������������������m1�IJ�ֵС��0.001 g c �������һ�����Ը��������Һ����Һ����ɫ��Ϊ�Ϻ�ɫ���Ұ���Ӳ���ɫ �����������Һ��ϴ2~3�� 20.20 mL
��������
ʵ���м��������BaCl2��Һ��Ŀ����ʹ����������ܳ�ֳ���������Һ�еμ�̼������Һ����û�а�ɫ�������ɣ���֤��BaCl2��Һ���㣻��ϴ�ӵõ������ᱵ������ϴ�ӹ���ķ���ͬ���ˣ��ڹ������м�������ˮ����û�����壬Ȼ����ˣ�ϴ�Ӹɾ��ķ��������ݳ���������ܸ����е��Ȼ��������ʵ����������ӵĴ����������ȷ�����ɵ����ᱵ������ȫ��ɣ�������������m1�IJ�ֵС��0.001g������һ�����ʵ���Ũ�ȵ���Һʱ��Ҫ�õ�����ƿ�����������ձ��������õζ��ܣ��ݴ˴��⣻�ø��������Һ�ζ���������Һʱ���ڵζ��յ㣬��Һ����ɫ����ɫ�����ݵζ��ܵ�ʹ�÷�����֪���ζ�����װ��Һǰ�����ô�װҺ��ϴ�����ݵζ��ܹ��켰ͼʾ�ζ�����Һ��������ĵĸ��������Һ�����
(1)ʵ���м��������BaCl2��Һ��Ŀ����ʹ����������ܳ�ֳ���������Һ�еμ�̼������Һ����û�а�ɫ�������ɣ���֤��BaCl2��Һ���㣻ϴ�ӹ���ķ���ͬ���ˣ��ڹ������м�������ˮ������û���壬ʹ����Ȼ���£��ظ�23�Σ�ϴ�Ӹɾ��ķ��������ݳ���������ܸ����е��Ȼ�������������Ϊ�������ϴ�ӹ��˳�����Һ�еμ���������Һ���������ɣ�����������ϴ����
�ʴ�Ϊ��ʹ����������ܳ�ֳ�����̼���ƣ��ڹ������м�������ˮ������û���壬ʹ����Ȼ���£��ظ�23�Σ���������
(2) �жϲ�����������m1g�ܹ���Ϊʵ��ⶨֵ�������ʽ��������Ʒ����,�����ظ���ɡ���ȴ����������ɺ������������m1�IJ�ֵС��0.001g��
�ʴ�Ϊ���ظ���ɡ���ȴ������,����������m1�IJ�ֵС��0.001g��
(3) ��������һ�����ʵ���Ũ�ȵ���Һ�����У���Ҫʹ�õ������У�һ���ݻ�������ƿ�����������ʱ�õ����������ܽ������Ҫ���ձ��н��У�����ʱ��Ҫʹ�ý�ͷ�ιܣ�������Ҫ�������У�a.����ƿ��b.��������d.�ձ���e.��ͷ�ιܣ�һ�㲻��Ҫ��Ϊc.�ζ��ܣ�
�ʴ�Ϊ��c��
(4) �ø��������Һ�ζ���������Һʱ�����������һ�����Ը��������Һ����Һ����ɫ��Ϊ��ɫ���Ұ���Ӳ���ɫ����˵���Ѿ��ﵽ�ζ��յ㣬
(5) ���ݵζ��ܵ�ʹ�÷�����֪���ζ�����ע�����Ը��������Һ֮ǰ����������ˮϴ�������ñ����������Һ��ϴ23�Σ��ζ��ܵ�ÿ��С�̶�Ϊ0.10mL������ͼ2��ʾ��ͼI��ʾ������Ϊ2.40mL��ͼII��ʾ��ĩ����Ϊ22.60mL���ζ��ܹ����ĵĸ��������Һ�����Ϊ��(22.602.40)mL=20.20mL��
�ʴ�Ϊ�������������Һ��ϴ23�Σ�20.20mL��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����100 mL 0.1 mol��L-1�������[NH4Al(SO4)2]��Һ����ε���0.1 mol��L-1 Ba(OH)2��Һ������Ba(OH)2��Һ���V�ı仯�����������ʵ���n�ı仯��ͼ��ʾ��������˵������ȷ����
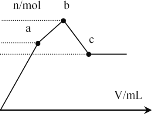
A.a�����Һ������
B.b�㷢����Ӧ�����ӷ���ʽ�ǣ�Al3++2SO42��+2Ba2++3OH��=Al(OH)3��+2BaSO4��
C.c����Һ�ʼ���
D.c�����Ba(OH)2��Һ�����Ϊ200 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
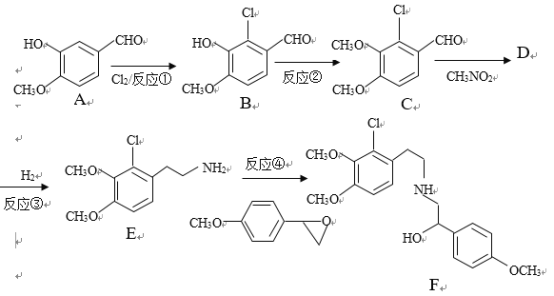
����Ŀ��ijҩ��ϳ��м���F�Ʊ�·�����£�
��֪��RCHO��R��CH2NO2![]()
 ��H2O
��H2O
(1)�л���A�ṹ�к��������ŵ�������__________________.
(2)��Ӧ���г�B�⣬����Ҫ�ķ�Ӧ��ͷ�Ӧ������___________________
(3)�л���D�Ľṹ��ʽΪ_____________________����Ӧ����1Ħ��D��Ҫ___Ħ��H2����ת��ΪE
(4)��Ӧ�ܵķ�Ӧ��![]() �ܶ���ͬ���칹�壬��д����������������һ��ͬ���칹��Ľṹ��ʽ_____________��
�ܶ���ͬ���칹�壬��д����������������һ��ͬ���칹��Ľṹ��ʽ_____________��
a.�ṹ�к�4�ֻ�ѧ������ͬ����ԭ��
b.�ܷ���������Ӧ
c.�ܺ�����������Һ��Ӧ
(5)��֪�������ϵ��Ȼ�Ϊ��λ��λ������![]()
![]()
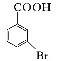 �����
��д����![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�![]() �ĺϳ�·������ͼ(���Լ���ѡ)_____________��
�ĺϳ�·������ͼ(���Լ���ѡ)_____________��
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ͻ�ѧʵ�������Чʵ�ֻ�ѧʵ����ɫ����Ҫ������ͼ��ʾ��һ��İ�ֽ�IJ���Ƭ�IJ�ͬλ�÷ֱ�μ�Ũ��Ϊ0.1mol/L��KI(��������Һ)��NaOH�ķ�̪(C20H14O4)��Һ��FeCl2(��KSCN)��Һ��һ�Σ���Բ�Ĵ�����һ��֥���С��KMnO4�ľ��壬��KMnO4�ľ����ϵμ�һ��Ũ���ᣬ�������ñ�����Ǻá��������������漰����Ԫ�أ��ش����⣺
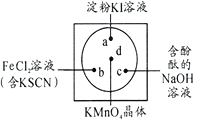
(1)KMnO4��MnԪ�صĻ��ϼ�Ϊ��_______________��Fe�����ڱ��е�λ���ǣ�_____________________��
(2)��������ԭ�Ӱ뾶��С��ԭ�ӹ���______ �ֲ�ͬ�����ĵ��ӣ�����������Ԫ���γɻ�����ĵ���ʽΪ��__________________��
(3)�����ѧ֪ʶ����a��c���Ŀ��ܳ��ֵ�����a��_____________________________��c��_________________________________��b�����ܷ�����Ӧ�����ӷ���ʽ��___________________________________________________________
(4)��֪��Һ�У���ԭ��ΪHSO3��I-��������ΪIO3��I2��SO42���ں�3molNaHSO3����Һ����μ���KIO3��Һ�������KIO3��������I2�����ʵ����Ĺ�ϵ������ͼ��ʾ��
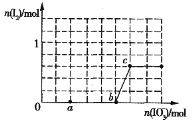
��a�㴦�Ļ�ԭ�����ǣ�____________(�����ӷ���)�� b����c�㷴Ӧ�����ӷ���ʽ�ǣ�_________________________________________
�ڵ���Һ�е�I-Ϊ0.5molʱ�������KIO3�����ǣ�____________________ mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Һ�ȴ����г�����NCl3����Һ����NCl3�����������ﵽ4%���Ͼ��б�ըΣ�ա�
(1)NCl3��ˮ������һ�ּ��������һ�־���Ư���Ե����ʡ�д���÷�Ӧ�Ļ�ѧ����ʽ________________________________________________��
(2)Ϊ�ⶨ�����е�NCl3��������һ������Ʒ��ͨ��������������NCl3(��ӦΪ��NCl3+4HCl��NH4Cl+3Cl2)������400 mL 20.0%(��=1.22g/cm3)�Ĺ�ҵNaOH��Һ�������е�Cl2��������պ�NaOH��Һ����51.4 g�����ⶨNCl3���չ���NH4+����Ϊ0.270 g��
��������ҵNaOH��Һ�����ʵ���Ũ��Ϊ____________��
�ڼ������Ʒ��NCl3���������������жϸ���Ʒ�Ƿ�ȫ��(д���������)____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)̼ԭ��2p�Dz��ϵ�2�����Ӳ���ͬ�ķ�����____��ѡ����ţ���
a������ b����������״ c����������չ���� d������״̬
14N ԭ����12Cԭ�ӱȣ��������������������࣬��ԭ�Ӱ뾶ȴ��С�������__________________��
(2)��(Be)�����������ơ�
�����¶��뼰�仯������ƶϿ϶�����ȷ����______��ѡ����ţ���
a�������Ũ�����жۻ� b���Ȼ������ᷢ����������
c���������Ӳ���� d������۵����þ
��д��BeCl2��Һ��Na2BeO2��Һ��Ϻ�Ӧ�����ӷ���ʽ____________________________��
(3)��BeCl2��Һ�������ɺ����ȣ���ʹ�����ڣ���ֱ�����⣬�ɵõ��������һ�ֵ������壬�������Ļ�ѧʽΪ_______��
(4)������(AlN)�㷺Ӧ���ڼ��ɵ�·�����Ʊ�ԭ���ǽ���������̼�ۻ�Ͼ��ȣ��ڳ��������ĵ������м�����1750�棬�������·�Ӧ��
2Al2O3(s)![]() 4Al(g)+3O2(g) ��
4Al(g)+3O2(g) ��
2C(s)+ O2(g)![]() 2CO(g) ��
2CO(g) ��
2Al(g)+N2(g)![]() 2AlN(s) ��
2AlN(s) ��
�Է�����Ӧ�ڶ��Ʊ�AlN������______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�ֺϳ�·�����£�
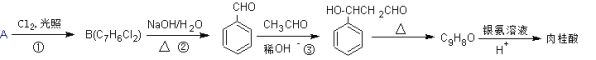
(1)��A������Ϊ_________����Ӧ����B�IJ���ƫ�ͣ���ԭ����________________________��
(2)��Ӧ�ڵĻ�ѧ����ʽΪ_________________________________________________��
(3)��Ӧ�۵ķ�Ӧ������________________��
(4)�����Ľṹ��ʽΪ__________________��
(5)��Ӧ�۵IJ���ͬ���칹���ж��֣����б�����ֱ����һ���������������_____�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������װ�г�������������ɣ���ԭ�����ۡ��Ȼ��ơ�̿�۵ȡ�����������
A.�������ⸯʴ
B.��������������������Ӧ
C.����1.12g���۵����������������������������336mL����״����
D.�������ȣ��Ӷ������¶ȣ����ʸ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���춡ϩ[CH2=C(CH3)2]����Ҫ�Ļ���ԭ�ϡ�
��֪��![]()
(1)�춡ϩ�ͱ�����һ�������·�Ӧ���ɶ��嶡����(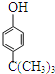 )���÷�Ӧ����_________��Ӧ(������Ӧ������)��
)���÷�Ӧ����_________��Ӧ(������Ӧ������)��
(2)���嶡���Ӻͼ�ȩ�ڴ��������¿����������Ծۺ��д���÷�Ӧ�Ļ�ѧ����ʽ________________��
(3)д���������������Ķ��嶡���ӵ�����ͬ���칹��Ľṹ��ʽ________________________________��
�ٺ���ͬ�����ţ��ڲ����ڷ��ࣻ�۱����ϵ�һ�����ֻ��һ�֡�
(4)��֪���춡ϩ��һ��ͬ���칹��A������һϵ�б仯�ɺϳ����ʣ���ϳ�·����ͼ��
![]()
������1Ϊ_____________��
��д���ṹ��ʽ��A_____________��B____________��
(5)�춡ϩ�ɶ�������CH2=C(CH3)CH2C(CH3)3��д���ö����������__________���춡ϩ����ʱ���������������Ķ���ϩ������д������һ����״ϩ���Ľṹ��ʽ________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com