
����Ŀ��������һ����Ҫ�Ļ�����Ʒ��ʵ������������������ˮ��Һ�Ʊ������װ����ͼ��ʾ(���ȡ�����������̶�װ�þ�����ȥ)��

ʵ��������£�
�ٽ�һ�����ĵ���ˮ��Һ��������ƿ�У�
�ڿ��Ʒ�ӦҺ�¶���55��60�������£��߽�������μ�һ�����������������Ļ���(65%HNO3��98%H2SO4��������Ϊ2��1.5)��Һ��
�۷�Ӧ3h���ң���ȴ�����˺����ؽᾧ�ò��ᾧ�壻
������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6+12HNO3��3H2C2O4+9NO2��+3NO��+9H2O
C6H12O6+8HNO3��6CO2+8NO��+10H2O
3H2C2O4+2HNO3��6CO2+2NO��+4H2O
(1)��������Ƿ�ˮ����ȫ�����õ��Լ�Ϊ________��
(2)ʵ����������μӹ��죬�����²�������½�����ԭ����_________��
(3)װ��C����β�����գ���β����n(NO2)��n(NO)=1��1ʱ��������NaOH��Һ�ܽ�NO��ȫ�����գ�ԭ����_________(�û�ѧ����ʽ��ʾ)��
(4)����NaOH��Һ����β����Ƚϣ����õ���ˮ��Һ����β�������š�ȱ����________��
(5)�����ؽᾧ�ļ�ѹ���˲����У����ձ����������⣬������ʹ�����ڹ����β��ϵ�������_________��
���𰸡���ˮ �����¶ȹ��ߡ�����Ũ�ȹ�����C6H12O6��H2C2O4��һ�������� NO2+NO+2NaOH=2NaNO2+H2O �ŵ㣺���HNO3�����ʣ�ȱ�㣺NOx���ղ���ȫ ����©��������ƿ
��������
(1)���ݵ��۵����Է������
(2)����������Ǿ����л�ԭ�ԣ������¶ȣ���������Ũ�ȣ������������Խǿ��
(3)NO�е�Ԫ��Ϊ+2�ۣ�NO2�е�Ԫ��Ϊ+4�ۣ��ڼ��������£�������̬���з�Ӧ��
(4)����β���ijɷֺ��������ƺ͵��۵����ʽ��
(5)���ݼ�ѹ���˵��ص�������
(1)�����������ɫ�����Ѿ�ˮ��ĵ�����Һ�еμӼ��ε�Һ����Һ����ɫ����֤������û����ȫˮ�⣻��Һ������ɫ����֤��������ȫˮ�⣬�ʴ�Ϊ����ˮ��
(2)����Ϊ65%HNO3��98%H2SO4�Ļ��Һ�����Һ����ˮ���ȣ��¶ȸ��ܼӿ컯ѧ��Ӧ��������μӹ��죬����Ũ�ȹ�����C6H12O6��H2C2O4��һ�����������ʴ�Ϊ���¶ȹ��ߡ�����Ũ�ȹ�����C6H12O6��H2C2O4��һ����������
(3) NO�е�Ԫ��Ϊ+2�ۣ�NO2�е�Ԫ��Ϊ+4�ۣ��ڼ��������£����߷�����Ӧ����NaNO2����Ӧ�Ļ�ѧ����ʽΪNO+NO2+2NaOH=2NaNO2+H2O���ʴ�Ϊ��NO2+NO+2NaOH=2NaNO2+H2O��
(4)β��Ϊһ�������Ͷ����������ü����գ����ǽ�ת��Ϊ����������ȫ���գ�����õ���ˮ��Һ���գ�����������ˮ��Ӧ�������ᣬ�������ܼ�����������������NOx���ղ���ȫ���ʴ�Ϊ���ŵ㣺���HNO3�����ʣ�ȱ�㣺NOx���ղ���ȫ��
(5)��ѹ�����볣ѹ������ȣ��ŵ㣺�ɼӿ�����ٶȣ����ܵõ��ϸ���ij�����װ���ص㣺����©������б��ҪԶ������������ƿ�ij����죬��˲����ؽᾧ�ļ�ѹ���˲����У����ձ����������⣬������ʹ�����ڹ����β��ϵ������в���©��������ƿ���ʴ�Ϊ������©��������ƿ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯����Ի�����������Ӱ�졣
��1����֪���������е����仯��������·�Ӧ��
��N2(g)+O2(g) 2NO(g) ��H=+180 kJ/mol
��N2(g)+2O2(g) 2NO2(g) ��H=+68 kJ/mol
��2NO(g)+O2(g) 2NO2(g) ��H=_____kJ/mol
��2�����ڷ�Ӧ2NO(g)+O2(g) 2NO2(g)�ķ�Ӧ�������£�
��һ����2NO(g)![]() N2O2(g)(����ƽ��)
N2O2(g)(����ƽ��)
�ڶ�����N2O2(g)+O2(g)![]() 2NO(g)(����Ӧ)
2NO(g)(����Ӧ)
���пɽ�����Ϊ�ڶ�����Ӧ��Ӱ���һ����ƽ�⣬��һ����Ӧ�У�����=k1 ����c2(NO)���� ��=k1 ����c(N2O2)��k1����k1 ��Ϊ���ʳ����������¶�Ӱ�졣����������ȷ����_____
a��������Ӧ�������ɵ�һ����Ӧ���ʾ���
b��ͬһ�¶��£�ƽ��ʱ��һ����Ӧ��![]() Խ��Ӧ����̶�Խ��
Խ��Ӧ����̶�Խ��
c���ڶ�����Ӧ�����������ƽ��ת����Ҳ��
d���ڶ�����Ӧ�Ļ�ܱȵ�һ����Ӧ�Ļ�ܸ�
��3���������������̼���д����ķ�Ӧ���з�Ӧ![]() ��H=��87.0 kJ/mol����ϵ�����صIJ��ʺʹ����Ļ������¶ȵĹ�ϵ��ͼ1��ʾ��
��H=��87.0 kJ/mol����ϵ�����صIJ��ʺʹ����Ļ������¶ȵĹ�ϵ��ͼ1��ʾ��
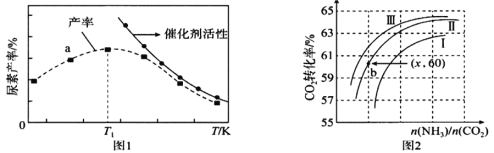
��a��_____(���ǻ���)����ƽ��״̬��T1֮�����ز����½���ԭ������� _____________��
��ʵ�������У�ԭ��������ˮ������ͼ2��ʾCO2��ת�����백̼��![]() ��ˮ̼��
��ˮ̼��![]() �ı仯��ϵ������������������Ӧ��ˮ̼��������_____�����b�㰱��ת����Ϊ30������x=______________��
�ı仯��ϵ������������������Ӧ��ˮ̼��������_____�����b�㰱��ת����Ϊ30������x=______________��
��4��N2H4��������ƽ�������֪25��ʱN2H4ˮ��Һ�������ԣ�N2H4+H2ON2H5++OH- K1=![]() ��N2H5++H2ON2H62++OH - K2=
��N2H5++H2ON2H62++OH - K2=![]() ��
��
��25��ʱ����N2H4ˮ��Һ�м���H2SO4����ʹc(N2H5+ )��c(N2H4 )��ͬʱc(N2H5+)>c(N2H62+)��Ӧ������ҺpH��Χ__________(�ú�a��bʽ�ӱ�ʾ)��
��ˮ����(N2H4��H2O)����������һˮ�ϰ��������ᷴӦ����������ʽ�Σ����εĻ�ѧʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������������������������ӦΪ��2NO2(g)+O3(g)![]() N2O5(g)+O2(g)����Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ����( )
N2O5(g)+O2(g)����Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ����( )
A | B | C | D |
|
|
|
|
�����¶ȣ�����Ӧ�����Ƿ��ȷ�Ӧ | 0~3s�ڣ���Ӧ����Ϊ��v(NO2)��0.2 mol��L-1��s-1 | t1ʱ�����������ƽ�������ƶ� | ��ƽ��ʱ�����ı�x����xΪc(O2) |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽ��.��H����ȷ��ʾ���ʵ�ȼ���ȵ��ǣ�������
A.CO(g) +![]() O2(g) ==CO2(g); ��H��-283.0 kJ/mol
O2(g) ==CO2(g); ��H��-283.0 kJ/mol
B.C(s) +![]() O2 ==CO(g); ��H��-110.5 kJ/mol
O2 ==CO(g); ��H��-110.5 kJ/mol
C.H2(g) +![]() O2(g)==H2O(g); ��H��-241.8 kJ/mol
O2(g)==H2O(g); ��H��-241.8 kJ/mol
D.2C8H18(l) +25O2(g)==16 CO2(g)+18 H2O(l); ��H��-1136 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Һ��������Խǿ,�絼��Խ��������0.1000 mol/L����ֱ�ζ�10.00 mLŨ�Ⱦ�Ϊ0.1000 mol/L��NaOH��Һ�Ͷ��װ�[(CH3)2NH]��Һ{���װ���ˮ�е����백����,��֪�ڳ�����Kb[(CH3)2NH��H2O]=1.6��10-4},���ô�������õζ���������Һ�ĵ絼����ͼ��ʾ������˵����ȷ����
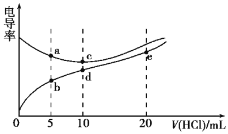
A.����ζ����װ�ʵ����ѡ���̪��ָʾ����ѡ��������С
B.d����Һ��:c(H+)>c(OH-)+c[(CH3)2NH2+]
C.b��c��d��e�ĵ����Һ��,ˮ�ĵ���̶�������d��
D.a����Һ��d�����Һ��Ϻ����Һ��: c(OH-) < c(H+) < c[(CH3)2 NH2+] <c(Na+)< c(Cl-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʷ����˵������ȷ����( )
A.����![]() ��
��![]() ��
��![]() ��
��![]() ������ij�����ж�
������ij�����ж�![]() ���������ֲ�ͬ��Ҳ�ɰ�ij�����ж�
���������ֲ�ͬ��Ҳ�ɰ�ij�����ж�![]() ���������ֲ�ͬ
���������ֲ�ͬ
B.�����ʷ���ʱ��һ�����ȷ��࣬�ٶ����������Ͳ���������
C.��״���෨��Ψһ�ܱ�ʾ���ʷ���ķ���
D.���塢��Һ����Һ������ͬ���ı�����������ֽ�����ʲ�ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ͭ�����ճ������г����Ľ���,���Ź㷺����;����ش���������:
(1)ijͬѧд������ԭ�ӵ�4�ֲ�ͬ״̬�ĵ����Ų�ͼ����������͵���___________(����ĸ)��������״̬B��״̬C����ԭ�ӹ���Ϊ___________����(����䡱�����ա�)��״̬D������ij�ּ���̬�����õ����Ų�ͼ�д�����Ҫ�Dz�����__________________________��
A. ![]()
B. ![]()
C. ![]()
D. ![]()
(2)K3[Fe(CN)6]��Һ�����ڼ���Fe2+,���ɳ��������ӷ���ʽΪ______________________________����CN- ��Ϊ�ȵ�����Ļ�������______��д���ƣ���
(3)��ʢ������ͭˮ��Һ���Թ�����백ˮ,�����γ�������,�����Ӱ�ˮ,�������ܽ�,�õ�����ɫ������Һ;�����뼫�Խ�С���ܼ�(���Ҵ�),����������ɫ�ľ��塣��ͭͬһ���ڵĸ���Ԫ�صĻ�̬ԭ���У�������������ͭԭ����ͬ��Ԫ�أ���ԭ����δ�ɶԵ�����Ϊ____��ʵ��ʱ�γɵ�����ɫ��Һ�е������ӵĽṹ��ʽΪ________��SO42-�����幹��Ϊ____,����ԭ�ӵ��ӻ��������Ϊ____��

(4)ij��Al-Fe�Ͻ�ľ�����ͼ��ʾ�����Ͻ���ܶ�Ϊ��g��cm-3,����Al��Fe����С����Ϊ___ pm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2O2����ȡ��������ˮ���������Ӧ���ǵ�ǰ��ѧ�о����ȵ㡣 �ش��������⣺

��1��������ͬ��������������������[��NH4��2S2O8]��ԭ����ͼ��ʾ����������������Ӧ��������_______�������ĵ缫��ӦʽΪ_________��
��2��100��ʱ���ڲ�ͬ�������Ӵ����£�����������24h�ķֽ��ʼ��±���
���� | ������/��mg��L-1�� | �ֽ���/% | ���� | ������/��mg��L-1�� | �ֽ���/% | |
�� | �� | 2 | Fe3+ | 1.0 | 15 | |
Al3+ | 10 | 2 | Cu2+ | 0.1 | 86 | |
Zn2+ | 10 | 10 | Cr3+ | 0.1 | 96 |
���ϱ����ݿ�֪����ʹ��������ֽⷴӦ��ܽ�������������_______�����˹�������ʱ����ѡ�õ���������Ϊ________�����ţ���
A ���� B ��ͭ C ���� D �����
��3���������������£�H2O2��һ�ִ��ֽ�������£�
H2O2��aq����Mn2+��aq��=OH��aq����Mn3+��aq����OH����aq�� ��H��a kJ/mol
H2O2��aq����Mn3+��aq����2OH����aq��=Mn2+��aq������O2- ��aq����2H2O��l�� ����b kJ/mol
OH��aq������O2-��aq��=O2��g����OH����aq�� ��H��c kJ/mol
��2H2O2��aq��=2H2O��l����O2��g������H��_________���÷�Ӧ�Ĵ���Ϊ________��
��4��298 Kʱ����10 mL a mol��L1 NaH2PO2��10 mL 2a mol��L1 H2O2��Һ��10 mL NaOH��Һ��ϣ�������Ӧ��H2PO2-��aq����2H2O2��aq����2OH��aq��![]() PO43-��aq����4H2O��l������Һ��c��PO43-���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
PO43-��aq����4H2O��l������Һ��c��PO43-���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
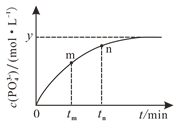
�����п��жϷ�Ӧ�ﵽƽ�����_______�����ţ���
a c��H2PO2-����y mol��L1
b ��Һ��pH���ٱ仯
c v��H2O2����2v��H2PO2-��
d c��PO43-��/c��H2PO2-�����ٱ仯
��tmʱv��_____tnʱv����������������С������������������
����ƽ��ʱ��Һ��pH��12����÷�Ӧ��ƽ�ⳣ��KΪ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
�ҹ���ѧ������ɹ��ϳ������������嵪��������(N5)6(H3O)3(NH4)4Cl����R���������ش��������⣺
��1����ԭ�Ӽ۲���ӶԵĹ������ʽ�������Ų�ͼ��Ϊ_____________��
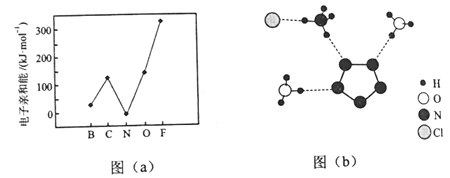
��2��Ԫ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��һ������ʱ���ų�������������һ�������ܣ�E1�����ڶ����ڲ���Ԫ�ص�E1�仯������ͼ��a����ʾ�����г���Ԫ���⣬����Ԫ�ص�E1����������������ԭ����___________����Ԫ�ص�E1�����쳣��ԭ����__________��
��3����X���������û�����R�ľ���ṹ����ֲ��ṹ��ͼ��b����ʾ��
�ٴӽṹ�Ƕȷ�����R�����������ӵ���֮ͬ��Ϊ_________����֮ͬ��Ϊ__________�������ţ�
A������ԭ�ӵ��ӻ�������� B������ԭ�ӵļ۲���Ӷ���
C������ṹ D�����ۼ�����
��R��������N5-�еĦҼ�����Ϊ________���������еĴ�м����÷���![]() ��ʾ������m���������γɵĴ�м�ԭ������n���������γɵĴ�м����������籽�����еĴ�м��ɱ�ʾΪ
��ʾ������m���������γɵĴ�м�ԭ������n���������γɵĴ�м����������籽�����еĴ�м��ɱ�ʾΪ![]() ������N5-�еĴ�м�Ӧ��ʾΪ____________��
������N5-�еĴ�м�Ӧ��ʾΪ____________��
��ͼ��b�������ߴ�����������ʾʽΪ��NH4+��N-H��Cl��____________��____________��
��4��R�ľ����ܶ�Ϊd g��cm-3����������������Ϊa nm�������к���y��[(N5)6(H3O)3(NH4)4Cl]��Ԫ���õ�Ԫ���������ΪM����y�ļ������ʽΪ______________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com