
����Ŀ��
�ҹ���ѧ������ɹ��ϳ������������嵪��������(N5)6(H3O)3(NH4)4Cl����R���������ش��������⣺
��1����ԭ�Ӽ۲���ӶԵĹ������ʽ�������Ų�ͼ��Ϊ_____________��
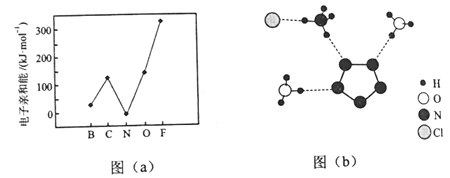
��2��Ԫ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��һ������ʱ���ų�������������һ�������ܣ�E1�����ڶ����ڲ���Ԫ�ص�E1�仯������ͼ��a����ʾ�����г���Ԫ���⣬����Ԫ�ص�E1����������������ԭ����___________����Ԫ�ص�E1�����쳣��ԭ����__________��
��3����X���������û�����R�ľ���ṹ����ֲ��ṹ��ͼ��b����ʾ��
�ٴӽṹ�Ƕȷ�����R�����������ӵ���֮ͬ��Ϊ_________����֮ͬ��Ϊ__________�������ţ�
A������ԭ�ӵ��ӻ�������� B������ԭ�ӵļ۲���Ӷ���
C������ṹ D�����ۼ�����
��R��������N5-�еĦҼ�����Ϊ________���������еĴ�м����÷���![]() ��ʾ������m���������γɵĴ�м�ԭ������n���������γɵĴ�м����������籽�����еĴ�м��ɱ�ʾΪ
��ʾ������m���������γɵĴ�м�ԭ������n���������γɵĴ�м����������籽�����еĴ�м��ɱ�ʾΪ![]() ������N5-�еĴ�м�Ӧ��ʾΪ____________��
������N5-�еĴ�м�Ӧ��ʾΪ____________��
��ͼ��b�������ߴ�����������ʾʽΪ��NH4+��N-H��Cl��____________��____________��
��4��R�ľ����ܶ�Ϊd g��cm-3����������������Ϊa nm�������к���y��[(N5)6(H3O)3(NH4)4Cl]��Ԫ���õ�Ԫ���������ΪM����y�ļ������ʽΪ______________��
���𰸡� 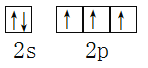 ͬ�������ź˵������������ԭ�Ӱ뾶��С���ʽ��һ�������ͷų��������������� N��2p�ܼ����ڰ����״̬������ȶ��������һ������ ABD C 5NA
ͬ�������ź˵������������ԭ�Ӱ뾶��С���ʽ��һ�������ͷų��������������� N��2p�ܼ����ڰ����״̬������ȶ��������һ������ ABD C 5NA ![]() ��H3O+��O��H��N ��NH4+����N��H��N
��H3O+��O��H��N ��NH4+����N��H��N 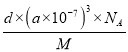
����������1��Nԭ��λ�ڵڶ����ڵ�VA�壬�۵������������ӣ��������Ų�ͼ��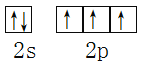 ��
��
��2������ͼ(a)��ͬ�������ź˵������������ԭ�Ӱ뾶��С���ʽ��һ�������ͷų���������������Ԫ�ص�2p�ܼ��ﵽ����״̬��ԭ������ȶ�������ʧȥ���ӣ�
��3���ٸ���ͼ(b)����������NH4����H3O����NH4������ԭ��N����4���Ҽ����µ��Ӷ���Ϊ(5��1��4��1)/2=0���۲���Ӷ���Ϊ4���ӻ�����Ϊsp3��H3O������ԭ����O������3���Ҽ����µ��Ӷ���Ϊ(6��1��3)/2=1���ռ乹��Ϊ���������Σ��۲���Ӷ���Ϊ4���ӻ�����Ϊsp3���ռ乹��Ϊ�����Σ������֮ͬ��ΪABD����֮ͬ��ΪC���ڸ���ͼ(b)N5���ЦҼ�����Ϊ5NA����������Ϣ��N5���Ĵ�Ӧ�DZ�ʾΪ�� ![]() ���۸���ͼ(b)���е�����ǣ���H3O+��O��H��N ��NH4+��N��H��N��
���۸���ͼ(b)���е�����ǣ���H3O+��O��H��N ��NH4+��N��H��N��
��4�������ܶȵĶ����У�d=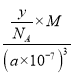 g/cm3�����y=
g/cm3�����y= ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ҹ�����ר�Һ�°Ľ����������������Ƽ����Ϊ�����Ƽҵ������ͻ�����ס�����������ͼ1:

��1������йط�Ӧ�Ļ�ѧ����ʽ
�ٳ����أ�NH3+CO2+H2O��NaCl=NaHCO3��NH4Cl
������¯��_______________��
��2�������Ƽ���ŵ�����У�����ȷ����_______��
A������ԭ��Ϊ��ʳ�Ρ�NH3��CO2
B���������Ȼ�刺�������
C�����������п�ѭ�����õ�����ֻ��CO2
D��ԭ�������ʸ�
ijʵ��С�飬��������װ��ͼ2ģ���������Ƽ���ĵ�һ����Ӧ��

��3������װ���нӿ�����˳��Ϊ______��
A.a��c��b��f��e��d B.a��d��b��f��e��c
C.b��d��a��e��f��c D.b��c��a��f��e��d
��4��D��Ӧѡ�õ�Һ��Ϊ______________��Ϊ�ⶨ��Ʒ����ijɷֺͺ�����������ʵ�飬�����Ʒ������ֻ��NaCI��NaHCO3���ʡ�
��5�������Ʒ�������Ƿ���NaCl����ȡ������������ˮ���ٵμ�___________�Լ���
��6���ζ����ⶨ�����Ʒ��NaHCO3�����ķ����ǣ�ȷ��ȡ������ƷWg��������ƿ�м�����ˮ�ܽ⣬��1��2�η�ָ̪ʾ������cmol/L��HCI��Һ�ζ�����Һ�ɺ�ɫ��Ϊ��ɫ��ָʾCO32-+H+=HCO3-��Ӧ���յ㣩������HCI ��Һ���ΪV1mL���ټ�1��2�μ���ָʾ����������HCl��Һ�ζ�����Һ�ɻ�ɫ��Ϊ��ɫ������HCI��Һ�����ΪV2mL������Ʒ��NaHCO3��������Ϊ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ʵķ�����ȷ����(����)
ѡ�� | �� | �� | �� | ���������� | ���������� |
A | Na2CO3 | H2SO4 | NaOH | SO2 | CO2 |
B | NaOH | HCl | NaCl | Na2O | NO |
C | KOH | HNO3 | CaCO3 | CaO | Mn2O7 |
D | NaOH | HCl | CaF2 | Na2O2 | SO2 |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A(C2H2)�ǻ����л�����ԭ�ϣ���A�Ʊ�����ϩ������ȩ��˳ʽ�������ϩ�ĺϳ�·��(���ַ�Ӧ������ȥ)��ͼ��ʾ��

�ش��������⣺
��1��A��������____________��B���еĹ�������____________��
��2���ٵķ�Ӧ������____________���ߵķ�Ӧ������____________��
��3��C��D�Ľṹ��ʽ�ֱ�Ϊ____________��____________��
��4�������ϩ�����������__________��ԭ�ӹ�ƽ�棬˳ʽ�������ϩ�Ľṹ��ʽΪ____________��
��5��д����A������ͬ�����ŵ������ϩ������ͬ���칹��(д�ṹ��ʽ)________________________________________________��
��6�����������ϩ�������ϳ�·�ߣ����һ����A����ȩΪ��ʼԭ���Ʊ�1��3-����ϩ�ĺϳ�·��____________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
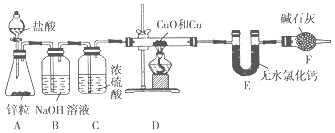
����Ŀ��Ϊ�ⶨijͭ������ͭ�Ļ������ͭԪ�ص�����������ijͬѧ�������ͼ��ʾʵ��װ�á�
�ش��������⣺
��1��)װ��A��ʢװ���������������Ϊ____________��
��2��ʵ��ʱװ��A�пɹ۲쵽������________________________________________ ��������Ӧ�����ӷ���ʽΪ__________________________________________________��
��3��װ��B��������_______________________________________________________��
��4���ڼ���װ��D֮ǰ��Ӧ�ý��е�һ��������________________________________��Ŀ����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ʊ��谱�����ƵĻ�ѧ����ʽΪCaCO3��2HCN===CaCN2��CO����H2����CO2�����ڷ�Ӧ��(����)
A. ��Ԫ�ر�������̼Ԫ�ر���ԭ B. HCN����������CaCO3�ǻ�ԭ��
C. CaCN2���������H2�ǻ�ԭ���� D. COΪ�������H2Ϊ��ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Al3+Ϊ1.08 g����������ѧʽ��KAl(SO4)212H2O������ˮ���200 mL��Һ���ڸ���Һ�м���0.1 mol/L Ba(OH)2��Һ200 mL����Ӧ����Һ��SO42�������ʵ���Ũ��Ϊ��������Һ����仯��
A. 0.6 molL��1 B. 0.3 molL��1 C. 0.15 molL��1 D. 0.075 molL��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�����������ͭƬ����Ƭ����Ƭ��пƬ�ֱ������ĸ�С�ձ��У�Ȼ��ֱ��������Ũ���ᣬ�ų�NO2����������
A��ͭƬ B����Ƭ C����Ƭ D��пƬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺
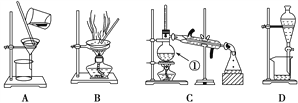
��1����װ��A��װ��B�ж��õ���������װ��A�в�������������________________��װ��B�в�������������________________________��ֹ����������Һ��ֲ����ȶ�������
��2��װ��C�Тٵ�������____________����ȴˮ�ķ�����____________��װ��D�ڷ�ҺʱΪʹҺ��˳�����£�Ӧ���еľ��������____________________________________��
��3�����Ȼ�����Һ�еõ��Ȼ��ƹ��壬ѡ��װ��______(�����װ��ͼ����ĸ����ͬ)����ȥ����ˮ�е�Cl�������ʣ�ѡ��װ��________����������ˮ��Cl���Ƿ�����ķ���Ϊ;ȡ������ƿ�е�ˮ�ڽྻ�Թ��У��μ�_________��Һ����������ɫ��������Cl���ѳ���;�ӵ�ˮ�з����I2��ѡ��װ��________���÷��뷽��������Ϊ________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com