
 ��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ����������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
��ѧ������ȤС��ѧ����ʵ��������ȡ����ϩ�г����������Ķ���������ʦ�������Dz��������Լ����������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A���þ����������Ӿ��� |
| B������Ļ�ѧʽΪBa2O2 |
| C���þ��徧���ṹ��CsCl���� |
| D����ÿ��Ba2+��������������Ba2+����12�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | V ��Na2CO3��/mL | ������� | ��� | ��Ӧ�¶�/�� | ������� | |
| 1 | 2.8 | �ࡢ��ɫ | 1 | 40 | �ࡢ��ɫ | |
| 2 | 2.4 | �ࡢ��ɫ | 2 | 60 | �١�dz��ɫ | |
| 3 | 2.0 | �϶ࡢ��ɫ | 3 | 75 | �϶ࡢ��ɫ | |
| 4 | 1.6 | ���١���ɫ | 4 | 80 | �϶ࡢ��ɫ��������ɫ�� |
| x |
| y |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��24 | B��18 | C��19 | D��29 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
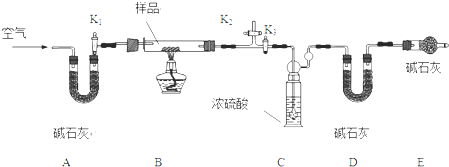
 ��������ͼ��ʾ��װ����ȡ����������
��������ͼ��ʾ��װ����ȡ�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���ϳ�·�����£�
���ϳ�·�����£�

| H2O2 |
 ��R-����������
��R-�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A����Ϊ
| ||
B��C1=
| ||
| C��d=a+17b | ||
| D���������Ӧ��ʣ������Ϊ��C1V1-b��mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
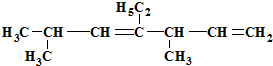 �������ǣ�������
�������ǣ�������| A��2��5-����-4-�һ�-3��6-����ϩ |
| B��1��1��4-����-3-�һ�-2��5-����ϩ |
| C��3��6-����-4-�һ�-1��4-����ϩ |
| D��2��5-����-4-�һ�-3��6-����ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ũ�������ǿ�����ԣ�ϡ������������ |
| B��Ũ����ε������ϣ�������ɰ�ɫ��ĩ��������Ũ�������ˮ�� |
| C��ϡ��Ũ����ʱӦ��Ũ���������ձ���������ע��ʢ��ˮ���ձ��У������Ͻ��� |
| D��Ũ������ͭ�ķ�Ӧ�У�Ũ���������ǿ������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com